
Concept explainers
Interpretation:
The pH of the titration of
Explanation of Solution
Given,
Titration of
Addition of
Given system is titration of
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 0.25 ml = 1.25 ml (or) 0.0625 L
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 0.50 ml = 1.50 ml (or) 0.0015 L
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | 0 | Excess |
The total volume of the given solution is 1 ml + 1.2 ml = 2.20 ml (or) 0.0022 L
The resulting solution is neutral. Therefore,
Hence,
The concentration of
Addition of
Given system is titration of
The given titration is shown below,
Moles of
Moles of
ICE table:
| S (mol) | 0 | Excess | ||
| R (mol) | ||||
| F(mol) | 0 | Excess |
The total volume of the given solution is 1 ml + 1.5 ml = 2.5 ml (or) 0.0025 L
The concentration of
The titration curve is given below,
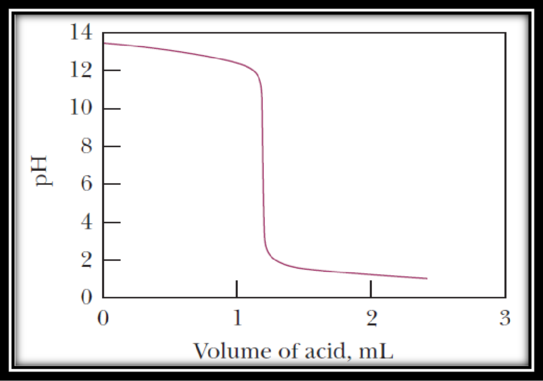
Figure 1
Want to see more full solutions like this?
Chapter 16 Solutions
Chemistry: Principles and Practice
- Calculate the pH during the titration of 50.00 mL of 0.100 M Sr(OH)2 with 0.100 M HNO3 after 0, 50.00, 100.00, and 150.00 mL nitric acid have been added. Graph the titration curve and compare with the titration curve obtained in Exercise 16.22.arrow_forwardCalculate the pH change when 10.0 mL of 0.100-M NaOH is added to 90.0 mL pure water, and compare the pH change with that when the same amount of NaOH solution is added to 90.0 mL of a buffer consisting of 1.00-M NH3 and 1.00-M NH4Cl. Assume that the volumes are additive. Kb of NH3 = 1.8 × 10-5.arrow_forwardWhich of the acid-base indicators discussed in this chapter would be suitable for the titration of (a) HNO3 with KOH. (b) KOH with acetic acid. (c) HCl with NH3. (d) KOH with HNO2. Explain your answers.arrow_forward
- Calculate the pH after 0.010 mole of gaseous HCl is added to 250.0 mL of each of the following buffered solutions. a. 0.050 M NH3/0.15 M NH4Cl b. 0.50 M NH3/1.50 M NH4Cl Do the two original buffered solutions differ in their pH or their capacity? What advantage is there in having a buffer with a greater capacity?arrow_forwardRepeat the procedure in Exercise 61, but for the titration of 25.0 mL of 0.100 M pyridine with 0.100 M hydrochloric acid (Kb for pyridine is 1.7 109). Do not calculate the points at 24.9 and 25.1 mL.arrow_forwardConsider die titration of 50.0 mL of 0.10 M H3A (Ka1 = 5.0 104, Ka2 = 1.0 108, Ka3 = 1.0 1012) titrated by 0.10 M KOH. a. Calculate the pH of the resulting solution at 125 mL of KOH added. b. At what volume of KOH added does pH = 3.30? c. At 75.0 mL of KOH added, is the solution acidic or basic?arrow_forward
- A buffer is prepared by dissolving 0.0250 mol of sodium nitrite, NaNO2, in 250.0 mL of 0.0410 M nitrous acid, HNO2. Assume no volume change after HNO2 is dissolved. Calculate the pH of this buffer.arrow_forwardConsider 1000. mL of a 1.00 104-M solution of a certain acid HA that has a Ka value equal to 1.00 104. How much water was added or removed (by evaporation) so that a solution remains in which 25.0% of HA is dissociated at equilibrium? Assume that HA is nonvolatile.arrow_forwardA 0.400-M solution of ammonia was titrated with hydrochloric acid to the equivalence point, where the total volume was 1.50 times the original volume. At what pH does the equivalence point occur?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





