
Concept explainers
We now continue the introduction of
To make your own reaction roadmap for Chapters 15–18, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized piece of paper filled out in landscape orientation.
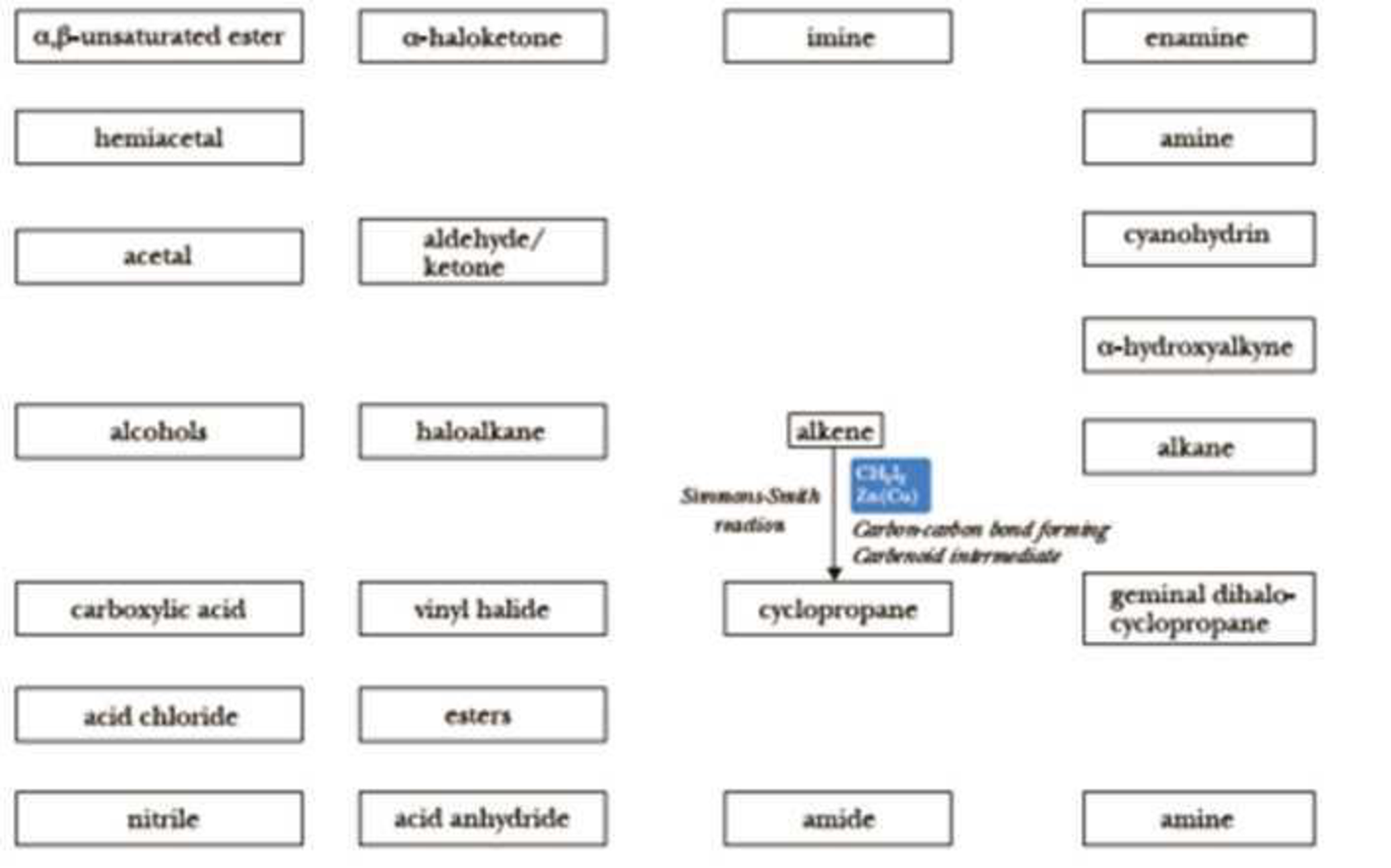
Refer to the “Study Guide” section of this chapter. Draw arrows between functional groups to account for each reaction. Write the reagents required to bring about each reaction next to the arrow. Then record any regiochemistry or stereochemistry considerations relevant to the reaction. You should also record any key aspects of the mechanism, such as formation of a carbocation intermediate, as a helpful reminder. It is important to keep track of all reactions that make carbon-carbon bonds, because these will help you build large molecules from smaller fragments.
On the above organic chemistry reaction roadmap template, the information for the Simmons-Smith reaction, the seventh reaction in the “Study Guide” section has been added to help you get started. For this reaction roadmap, do not write an arrow for reactions 1, 2, and 4 explicitly, because these are considered reagents, which are prepared immediately prior to use. A reaction roadmap is used to indicate interconversion of molecules with more stable functional groups. Appendix 10 contains a series of roadmaps for different sections of the book, but you should use those for reference only after you have completed your own.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: Atoms First
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry For Changing Times (14th Edition)
Organic Chemistry
Chemistry & Chemical Reactivity
- We now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 2023. To make your own roadmap for Chapters 2023, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation. We now continue the use of organic chemistry reaction roadmaps. Because of the unique nature of the new reactions presented, we recommend that you make a new roadmap only for Chapters 2023. To make your own roadmap for Chapters 2023, take a blank sheet of paper and write the following functional groups in the orientations shown. Fill the entire sheet of paper and leave plenty of room between functional groups. Most students find it helpful to use a poster-sized sheet of paper filled out in landscape orientation.arrow_forwardWrite the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapter 20 reaction roadmap for these.arrow_forwardWrite the products of the following sequences of reactions. Refer to your reaction roadmaps to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need your old Chapters 611, Chapters 1518, and Chapter 19 roadmaps along with your new Chapters 2021 roadmaps for these.arrow_forward
- Write the products of the following sequences of reactions. Refer to your reaction roadmap to see how the combined reactions allow you to navigate between the different functional groups. Note that you will need both your old Chapters 611 roadmap and your new Chapters 1517 roadmap for these.arrow_forwardA newer generation of antipsychotics, among them clozapine, are now used to treat the symptoms of schizophrenia. These drugs are more effective than earlier drugs in improving patient response in the areas of social withdrawal, apathy, memory, comprehension, and judgment. They also produce fewer side effects such as seizures and tardive dyskinesia (involuntary body movements). In the following synthesis of clozapine, Step 1 is an Ullmann coupling, a type of nucleophilic aromatic substitution that uses a copper catalyst. (a) Show how you might bring about formation of the amide in Step 2. (b) Propose a reagent for Step 3. (c) Propose a mechanism for Step 4. (d) Is clozapine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardThese synthesis reactions can be carried out in three or fewer steps. Fill in the reagents required for each step. If a step is not needed, write an X for that step.arrow_forward
- For the following synthesis scheme, fill in the missing molecules. Note, you do not understand all of this chemistry yet, but soon you will and then you will be responsible for the entire sequence of synthetic reactions! Even if you have not seen all the chemistry, please take a moment to see what kind of transformations are occuring in each step of the synthesis.arrow_forward3. Propose a reasonable mechanism for the reaction. Remember to: 1) Draw out the Lewis structure 2) Use arrows to indicate electron flow, clearly identifying the bond making and bond breaking steps. 3) Propose reasonable intermediates for the reaction conditions. Hint: One of the intermediates is a "dienol" which leads to an interesting tautomerization. For practice include the tautomerization in your mechanism. (Ph= phenyl = C6Hs) Ph 4. a. b. C. d. OH + H₂O H,CO. f. Ph cat. H₂SO4 For the reactions below, choose an appropriate reagent for the transformation. HO HICO. Reagent OH S HO Reagent + CO₂ Reagent Reagent Reagent H Ph Reagent -Pharrow_forwardAlcohols are acidic in nature. Therefore, a strong base can abstract the acidic hydrogen atom of the alcohol in a process known as deprotonation. The alcohol forms an alkoxide ion by losing the proton attached to the oxygen atom of the hydroxyl ( -OH) group. The alkoxide formed can act as a base or a nucleophile depending on the substrate and reaction conditions. However, not all bases can abstract the acidic proton of alcohols and not all alcohols easily lose the proton. Deprotonation depends on the strength of the base and the acidity of the alcohol. Strong bases, such as NaNH2, can easily abstract a proton from almost all alcohols. Likewise, more acidic alcohols lose a proton more easily. Determine which of the following reactions would undergo deprotonation based on the strength of the base and the acidity of the alcohol. Check all that apply. ► View Available Hint(s) CH3CH,OH + NH3 →CH,CH,O-NH CH3 CH3 H3C-C-H+NH3 → H3 C-C-H OH O-NH CH3CH2OH + NaNH, → CH3CH,O-Na* + NH3 CHC12 Cl₂…arrow_forward
- In both examples below the reactants shown are combined to bring about a nucleophilic substitution (Sy1, Sy2) and/or elimination (E1, E2) reaction. What is the major reaction that takes place in each case? CI CH2CI NaOH H20 SN2 E2 mixture of SN1 and E1 CH3 CI CH3OH CH3arrow_forwardStarting with benzene and using any other reagents of your choice, design a synthesis for the following compound: COOH NO₂ COOH The target molecule above can be prepared by treating benzene with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A Mel, AlCl3 B KMnO4, NaOH, heat (followed by H3O+ workup) C HCI, Zn D E F (CH3)2CHCI, AICI 3 HNO3, H2SO4 Fuming H2SO4arrow_forward6. Both acid-catalyzed hydration and oxymercuration-demercuration are addition reactions that add a hydroxyl group (--OH) and a hydrogen atom across a t-bond to make a Markovnikov product. Because of this, both reactions can often end with the same major organic product. 3 Explain in 1-3 complete sentences why substrate A leads to the same product no matter the conditions and why substrate B leads to two different products based on reaction conditions. 4 10 A ER A % H₂SO4 H₂O 1. Hg(OAc)2 2. NaBH4 當 5 OH Q Search T (racemic) OH (racemic) 6 & B B H₂SO4 H₂O 1. Hg(OAc)₂ 2. NaBH4 OH (racemic) lyi Oarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
