
Interpretation:
The given cycloalkane undergoes reaction with the given Simmons-Smith reagent which is stereospecific and gives only the isomer shown. The reason has to be suggested.
Concept Introduction:
Simmons-Smith reaction: This is ultrasonication improve the rate of formation of these organic zinc compounds, as with many organometallic reactions occurring at a surface condition.
Example: The substance of a carbenoid a carbine like substance that converters
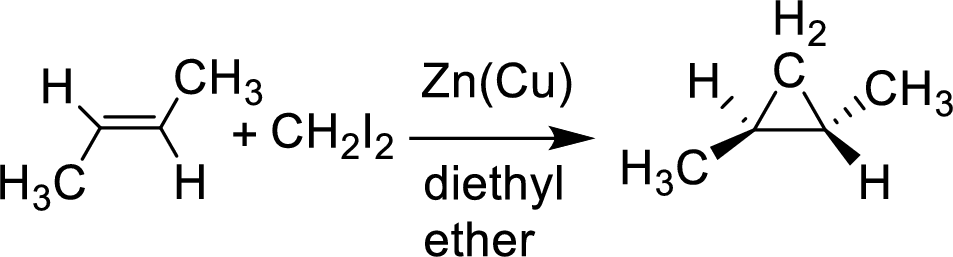
Addition Reaction:
- It is a type of reaction in which two reactants adding together to form a single product.
- It can be said as a reverse reaction of elimination reaction.
- It is a characteristic reaction of
alkane .
Hydrogenation reaction is an addition reaction in which addition of hydrogen to an unsaturated molecule occurs
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space.
Stereospecific reaction: reaction undergoes from a stereoisomer to a unique stereo isomeric product.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Organic Chemistry
- -Ocimene is a pleasant-smelling hydrocarbon found in the leaves of certain herbs. It has the molecular formula C10H16 and a UV absorption maximum at 232 nm. On hydrogenation with a palladium catalyst, 2,6-dimethyloctane is obtained. Ozonolysis of -ocimene, followed by treatment with zinc and acetic acid, produces the following four fragments: (a) How many double bonds does -ocimene have? (b) Is -ocimene conjugated or nonconjugated? (c) Propose a structure for -ocimene. (d) Write the reactions, showing starting material and products.arrow_forwardBromocyclopentane is more reactive than bromocyclohexane when heated with sodium iodide in acetone. Explain this difference in reactivity.arrow_forwardCyclobutane fracts with bromine to give bromocyclobutane, but bicyclobutane reacts with bromine to give 1,3-dibromocyclobutane. Account for the differences between the reactions of thee two compounds.arrow_forward
- The electrophilic addition of HBr to 3-cyclohexylbut-1-ene gives a mixture of two constitutional isomers. These two isomers can also be produced from 3-cylcohexybut-2-ene, but one of which requires different reaction conditions; the electrophilic addition of HBr to 3-cylcohexybut-2-ene produces one of these isomers while the electrophilic addition of HBr to 3-cylcohexybut-2-ene, in the presence of peroxides, produces the other one. Draw the structure for the isomer product that can result from the reactants 3-cyclohexylbut-1-ene and 3-cylcohexybut-2-ene using different reaction conditions. Part 1 of 2 Click and drag to start drawing a structure. C 8 DY 000 8: >arrow_forwardExplain this observation: Ethyl 3-phenylpropanoate (C6H5CH2CH2CO2CH2CH3) reacts with electrophiles to afford ortho- and para-disubstituted arenes, but ethyl 3-phenylprop-2-enoate (C6H5CH=CHCO2CH2CH3) reacts with electrophiles to afford meta- disubstituted arenes.arrow_forwardWhat is the major product obtained from treating an excess of each of the following compounds with Cl2 in the presence of ultraviolet light at room temperature? Disregard stereoisomers.arrow_forward
- Two isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H₂O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. Which of the following isomeric pairs best match this data? a. Isomer A is cyclopentene and isomer is 1-pentyne O b. Isomer A is cyclopentene and isomer B is 1-methylcyclobutene c. Isomer A is cyclopentene and isomer B is 3-methylcyclobutene d. Isomer A is 1-methylcyclobutene and isomer B is 3-methylcyclobutenearrow_forwardThe following alkene is treated with one equivalent of N-Bromosuccinimide in dichloromethane in the presence of light to give bromination product(s). Draw a line-angle formula for each product formed.arrow_forwardTwo isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. Which of the following isomeric pairs best match this data?arrow_forward
- Two isomers, A and B, of molecular formula C5H8 undergo catalytic hydrogenation with hydrogen gas and palladium on carbon to form the same C5H10 product. On ozonolysis followed by treatment with hydrogen peroxide (H2O2), isomer A gave a product of molecular formula C5H8O4 that has two carboxylic acid groups in it whereas isomer B gave a product of molecular formula C5H8O3 that contains a carboxylic acid group and a ketone group. What is the isometric pair of A and B that corresponds?arrow_forwardThe Wittig reaction can be used for the synthesis of conjugated dienes, as, for example, 1-phenyl-1,3-pentadiene. -CH=CHCH CHCH, 1-Phenyl-1,3-pentadiene Propose two sets of reagents that might be combined in a Wittig reaction to give this conjugated diene.arrow_forwardHow many alkenes yield 2,2,3,4,4−pentamethylpentane on catalytic hydrogenation?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

