
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 48P
A compound
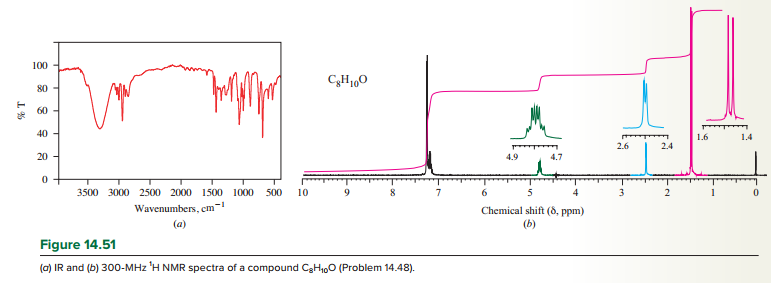
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Identify the structures of isomers A and B (molecular formula C8H9Br).
Three isomeric compounds, A, B, and C, all have molecular formula C8H11N. The 1H NMR and IR spectral data of A, B, and C are given below. What are their structures?
Compound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C-NMR shows five signals. The mass spectrum of A shows a peak at m/z = 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively.
- Identify what atom X is, explaining your reasoning
- Identify Compound A, explaining your reasoning
Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively.
- Identify compound B, explaining your reasoning
Compound B is treated with sodium ethoxide to generate compound C. The 1H-NMR spectrum of C shows…
Chapter 14 Solutions
Organic Chemistry - Standalone book
Ch. 14.3 - Prob. 1PCh. 14.3 - Prob. 2PCh. 14.4 - The 1H NMR signal for bromoform (CHBr3) appears at...Ch. 14.5 - identify the most shielded and least shielded...Ch. 14.5 - (a) Assign the chemical shifts 1.6, 2.2, and 4.8...Ch. 14.5 - Assign the chemical shifts 1.1, 1.7, 2.0, and 2.3...Ch. 14.5 - Assign the chemical shifts 1.6, 4.0, 7.5, 8.2, and...Ch. 14.6 - The 300-MHz 1H NMR spectrum of 1,4-dimethylbenzene...Ch. 14.6 - Prob. 9PCh. 14.6 - How many signals would you expect to find in the...
Ch. 14.7 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.8 - Describe the appearance of the 1H NMR spectrum of...Ch. 14.11 - Prob. 13PCh. 14.11 - Prob. 14PCh. 14.12 - Hydrogen bonding between the oxygen of dimethyl...Ch. 14.14 - Prob. 16PCh. 14.15 - The 13C NMR spectrum of 1-bromo-3-chloropropane...Ch. 14.15 - Consider carbons x, y, and z in p-methylanisole....Ch. 14.15 - Prob. 19PCh. 14.16 - To which of the compounds of Problem 14.16 does...Ch. 14.18 - DEPT spectra for a compound with the formula...Ch. 14.20 - Vibrational frequencies are sensitive to isotopic...Ch. 14.21 - Prob. 23PCh. 14.22 - Prob. 24PCh. 14.23 - Prob. 25PCh. 14.23 - Which one of the C5H8 isomers shown has its max at...Ch. 14.24 - Knowing what to look for with respect to isotopic...Ch. 14.24 - The base peak appears at m/z105 for one of the...Ch. 14.24 - Mass spectra of 1-bromo-4-propylbenzene and...Ch. 14.25 - Prob. 30PCh. 14 - Each of the following compounds is characterized...Ch. 14 - Deduce the structure of each of the following...Ch. 14 - From among the isomeric compounds of molecular...Ch. 14 - The H1NMR spectrum of fluorene has signals at 3.8...Ch. 14 - Prob. 35PCh. 14 - H1NMR spectra of four isomeric alcohols with...Ch. 14 - Prob. 37PCh. 14 - We noted in Section 14.13 that an NMR spectrum is...Ch. 14 - Identify each of the C4H10O isomers on the basis...Ch. 14 - A compound (C3H7ClO2) exhibited three peaks in its...Ch. 14 - Label nonequivalent carbons in the following...Ch. 14 - Compounds A and B are isomers of molecular formula...Ch. 14 - C13 NMR spectra for four isomeric alkyl bromides...Ch. 14 - Prob. 44PCh. 14 - Prob. 45PCh. 14 - Identify the C3H5Br isomers on the basis of the...Ch. 14 - Prob. 47PCh. 14 - A compound (C8H10O) has the IR and H1NMR spectra...Ch. 14 - Deduce the structure of a compound having the...Ch. 14 - Figure 14.53 presents IR, H1NMR, C13NMR and mass...Ch. 14 - H1NMR, C13NMR, IR, and mass spectra are shown for...Ch. 14 - 1H NMR and IR spectra for a compound with the...Ch. 14 - FriedelCraftsalkylation of benzene with...Ch. 14 - Prob. 54DSPCh. 14 - Prob. 55DSPCh. 14 - Prob. 56DSPCh. 14 - Prob. 57DSPCh. 14 - Prob. 58DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 16-19 2-Me thy 1 propane (bp -12°C), 2-propanol (bp 82°C), and 2-propanamine (bp 32°C) all have approximately the same molecular weight, yet their boiling points are quite different. Explain the reason for these differences.arrow_forward17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C) have about the same molecular weight, yet their boiling points differ by almost 50°C. Explain this fact.arrow_forwardSee image belowarrow_forward
- Identify the structures of D and E, isomers of molecular formula C6H12O2, from their IR and 1H NMR data. Signals at 1.35 and 1.60 ppm in the 1H NMR spectrum of D and 1.90 ppm in the 1H NMR spectrum of Eare multiplets.a. IR absorption for D at 1743 cm−1b. IR absorption for E at 1746 cm−1arrow_forwardCompound A has molecular formula C7H7X. Its ¹H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C- NMR shows five signals. The mass spectrum of A shows a peak at m/z 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoning Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The ¹H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoning Compound B is treated with sodium ethoxide to generate compound C. The ¹H-NMR spectrum of C shows…arrow_forwardExplain why phenol (C6H50H) is substantially more acidic than methanol (CH3OH), but benzoic acid (C6H5CO2H) is not much more acidic than acetic acid (CH3CO2H). Нас — ОН ОН H3C- ОН OH pKa = 15.5 pKa = 10.0 pk = 4.75 pk = 4.2arrow_forward
- Which type of compound typically give 3 peaks ("bands") between approx. 1500-1600 cm in an IR spectrum? Select one: Carbonates Esters Alkaloids Aromatic compoundsarrow_forward3) For the following questions draw the structure of the compound based off of the molecular formula and NMR that is given. a) CHa Draw the structure of the compound. 12H 8H CH PPM b) CioH12O2 Draw the structure of the compound. t 3H d, 2H d. 2H L 2H brs, 1H 12 PPM c) CH10O3 (IR stretches: strong signals at 1750 and 1730 cm") Don't worry about stereochemistry of the product. Draw the structure of the compound. d. 3H s, 1H t 3H 9. 2H q. 1H 10 6. PPM 2.arrow_forwardThe 'H NMR and 13C spectra of a compound with a molecular formula of C6H12O2 are shown below. 1. Name the compound in the textbox below. 2. Draw a possible structure for this compound. 1Η NMR 2H 2H 2H 3HPPM 3H 13C NMR 220 200 100 140 100 120 PPMarrow_forward
- 5 The compounds labeled benzophenone-3 (CH,O,) and benzophenone-5 (CHNAO,S) are found in certain sunscreens. Would you expect a sunscreen made with benzophenone-3 or benzophenone-5 to be more waterproof? Explain your choice.arrow_forwardCompound X (molecular formula C10H12O) was treated with NH2NH2, −OH to yield compound Y (molecular formula C10H14). Based on the 1HNMR spectra of X and Y given below, what are the structures of X and Y?arrow_forwardProtons on a carbon atom located adjacent to the carbonyl group generally absorb in the range of 1-2.5 ppm. Protons on a carbon atom located adjacent to an oxygen atom generally absorb in the range of 3.5-5 ppm. What is the structure of the ester from the Proton NMR spectrum?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY