
(a)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
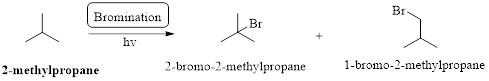
(b)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
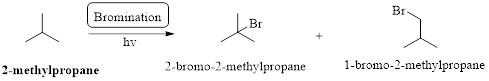
(c)
Interpretation:
The major product obtained by the reaction of excess of given compound with bromine in presence of light at room temperature should be identified.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Bond strength is depends on the formation of the radical, if the radical is involving in resonance which is weakest bond strength.
In a halogenation reaction, one or more halogen atoms are introduced into an organic compound. Generally, these reactions are initiated in the presence of light or heat.
Bromination:
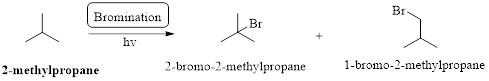
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Essential Organic Chemistry, Global Edition
- What reaction presented in this chapter is occurring in the following equation? Explain the resulting stereochemistry of the reaction.arrow_forwardRank the following hydrocarbons in order of decreasing rates of light-catalyzed bromination. Give the theoretical explanation for the order. Also, give the major bromination product for each.arrow_forwardTwo products are possible when pent-2-ene is treated with HBr. Write the structures of the possible products, and explain why they are made in about equal amounts.arrow_forward
- Elimination of HBr from 2-bromobutane affords a mixture of but-1-ene and but-2-ene. With sodium ethoxide as base, but2-ene constitutes 81% of the alkene products, but with potassium tert-butoxide, but-2-ene constitutes only 67% of the alkene products. Offer an explanation for this difference.arrow_forwardWhich of the following alkenes will undergo a carbocation rearrangement in their reaction with ethanol in the presence of a small amount of strong acid?arrow_forwardCompounds X and Y are both C7H15Cl products formed in the radical chlorination of 2,4-dimethylpentane. Base-promoted E2 elimination of X and Y gives, in each case, a single C7H₁4 alkene. Both X and Y undergo an SN2 reaction with sodium iodide in acetone solution to give C7H15l products; in this reaction Y reacts faster than X. What is the structure of X? • Do not use stereobonds in your answer. • In cases where there is more than one possible structure for each molecule, just give one for each. . Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu. наarrow_forward
- Each of the following reactions has been described in the chemical literature and gives a single organic product in good yield. Identify the product of each reaction.arrow_forwardPredict the coupling products of organometallic substitutions, and use them in syntheses.arrow_forwardCompounds X has the formula C7H15Cl; Y is C7H15Br.X undergoes base-promoted E2 elimination to give a single alkene product Z. Y likewise reacts under similar conditions to give a single alkene product that is isomeric with ZCatalytic hydrogenation of Z affords 3-ethylpentane.X readily reacts in SN2 fashion with sodium iodide in acetone. Y does not undergo a similar SN2 reaction. Propose structures for X and Y.arrow_forward
- Rank the compounds in order of increasing reactivity in electrophilic aromatic substitution. Briefly explain your answer. CóH5OH, C6H5SO3H, C6H5CIarrow_forwardWhen butane reacts with Br₂ in the presence of Cl₂, both brominated and chlorinated products are obtained. Under such conditions, the usual selectivity of bromination is not observed. In other words, the ratio of 2-bromobutane to 1-bromobutane is very similar to the ratio of 2-chlorobutane to 1-chlorobutane. Can you offer and explanation as to why we do not observe the normal selectivity expected for bromination? Chlorine radicals perform the first propagation step (hydrogen abstraction) comparison to bromine radicals. Under these conditions in radicals form easily in the presence of chlorine radicals. Subsequently, the resulting radicals can react with bromine in a second propagation step to yield monobrominated products.arrow_forwardHeterocyclic compounds can undergo nitration reactions with some regioselectivity, giving products in majority. From the following reaction give the raw material from which it is formed and the mechanism under which the transformation takes place.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

