
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.5, Problem 8P
Which
a. 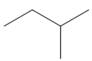 b.
b. 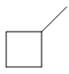 c.
c. 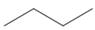
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which reaction will produce this alkene as the major product?
A. A
B. B
C. Both
D. Neither
(a)
(b)
Br
NaOCH3
Δ
H₂C
P(Ph)3
Substitution and Elimination (Please draw the structures involved in the reactions)
A. Which will react more rapidly via SN1? 1-bromo-2,2-dimethylpropane or 2-bromo-2-
methylbutane? Explain.
B. Which will react faster via SN2? 1-chlorocyclohexane or 1-chloro-1-methylcyclohexane?
Explain.
What reagent/reagents is/are necessary to transform the starting molecule into the
desired product?
SO3H
?
Chapter 13 Solutions
Organic Chemistry (6th Edition)
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What reagent/reagents is/are necessary to transform the starting molecule into the desired product? ?. CH,CH•CHỔH CH3CHarrow_forwardWhat reagent/reagents is/are necessary to transform the starting molecule into the desired product? N. CH3 ? H3C CH3arrow_forward8. Explain WHY the reaction of alkene with water/alcohol require acid, while the reaction of an alkene with Br2 does not.arrow_forward
- ● Rank the following alkenes from most to least stable. A 11 B ||| MacBook Air C IVarrow_forwardDraw the products obtained from the SN2 reaction of a. 2-bromobutane and methoxide ion. b. (R)-2-bromobutane and methoxide ion. c. (S)-3-chlorohexane and hydroxide ion. d. 3-iodopentane and hydroxide ion.arrow_forward2) Which species has a reasonable leaving group attached to the carbonyl carbon? a. Acetonitrile b. Formaldehyde c. Acetone d. Acetyl chloride e. Bromobenzaldehydearrow_forward
- Q6. What is nucleophilic substitution reaction? Illustrate nucleophilic substitution 1 (SN1) mechanism and explain each step. Identify the differences between nucleophilic substitution 1 (SN,) and nucleophilic substitution 2 (SN2) reactions and explain why a tertiary carbocation is more favoured than a primary carbocation in SN, reactions.arrow_forward. Which of the following can be both formed from bromoethane and converted directly into ethanal? CH3CH2Br → X → CH3CHO a. CH3CH2OH b. CH3OCH3 c. CH3COOH d. H2C=CHBrarrow_forwardThe Diels–Alder reaction, a powerful reaction discussed in Chapter 14, occurs when a 1,3-diene such as A reacts with an alkene such as B to form the six-membered ring in C. a.Draw curved arrows to show how A and B react to form C. b.What bonds are broken and formed in this reaction? c.Would you expect this reaction to be endothermic or exothermic? d.Does entropy favor the reactants or products? e. Is the Diels–Alder reaction a substitution, elimination, or addition?arrow_forward
- What reactant converts an internal alkyne to an alkene with trans stereochemistry? a. H2/Ni b. none c. H2/Lindlar d.Na/NH3arrow_forward2. Explain the selectivity of the following reaction, which produces a single product despite both alkene carbons being equally substituted. H3C CF3 HBr CF3 H3C CF3 H3C Br- H3C only product -H CF3arrow_forwardH₂C CH₂ 2 c CH₂ 1. Reaction A is a radical process. The part of the mechanism that is represented above is called [blank1] would immediately follow [blank2] A. a. nucleophilic attack; hydrogen abstraction b. homolytic attack; hydrogen abstraction c. hydrogen abstraction; homolytic cleavage d. nucleophilic attack; homolytic cleavage 2. Radical mechanisms begins by which of the following events: a) homolytic cleavage b) heterolytic cleavage c) extreme heating or exposure to light irradiation. d) nucleophilic attack 3. Rank the following radicals from most stable to least stable: CH H₂C A CH₂ a) D > C>A>B b) B>D>A>C c) D> B>A>C d) C>A>B>D H₂C B CH₂ H₂C" CH₂ C CH3 H₂C CH D CH₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY