
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.5, Problem 8P
Which
a. 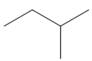 b.
b. 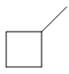 c.
c. 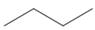
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain why radical bromination of p-xylene forms C rather than D ?
b) Explain why m-xylene undergoes nitration 100 times faster than p-xylene.
a.What carbon radical is formed by homolysis of the C–Ha bond in propylbenzene? Draw all reasonable resonance structures for this radical.
b.What carbon radical is formed by homolysis of the C–Hb bond in propylbenzene? Draw all reasonable resonance structures for this radical.
c. The bond dissociation energy of one of the C–H bonds is considerably less than the bond dissociation energy of the other. Which C–H bond is weaker? Offer an explanation.
Chapter 13 Solutions
Organic Chemistry (6th Edition)
Ch. 13.1 - Prob. 1PCh. 13.1 - Prob. 2PCh. 13.2 - Prob. 3PCh. 13.3 - Prob. 4PCh. 13.3 - Prob. 5PCh. 13.4 - Prob. 7PCh. 13.5 - Problem 15.8 Which bond in the each compound is...Ch. 13.6 - Prob. 9PCh. 13.6 - Prob. 10PCh. 13.7 - Prob. 11P
Ch. 13.7 - Prob. 12PCh. 13.8 - Prob. 13PCh. 13.8 - Prob. 14PCh. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 34PCh. 13 - 15.37 What alkane is needed to make each alkyl...Ch. 13 - 15.38 Which alkyl halides can be prepared in good...Ch. 13 - Prob. 37PCh. 13 - 15.40 Explain why radical bromination of p-xylene...Ch. 13 - a. What product(s) (excluding stereoisomers) are...Ch. 13 - Prob. 40PCh. 13 - 15.43 Draw the products formed when each alkene is...Ch. 13 - 15.44 Draw all constitutional isomers formed when...Ch. 13 - 15.45 Draw the organic products formed in each...Ch. 13 - Prob. 45PCh. 13 - 15.47 Treatment of a hydrocarbon A (molecular...Ch. 13 - 15.48 Draw the products formed in each reaction...Ch. 13 - 15.53 Consider the following bromination: .
a....Ch. 13 - 15.54 Draw a stepwise mechanism for the following...Ch. 13 - Prob. 57PCh. 13 - 15.57 Devise a synthesis of each compound from...Ch. 13 - Prob. 59PCh. 13 - Prob. 60PCh. 13 - 15.60 Devise a synthesis of each compound using ...Ch. 13 - Prob. 62PCh. 13 - Prob. 63PCh. 13 - 15.63 As described in Section 9.16, the...Ch. 13 - 15.64 Ethers are oxidized with to form...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Resveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inflammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.arrow_forwardResveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.arrow_forwardethers can often be prepared by Sn2 reaction of an alkoxide and alkylhalides, which is a better reaction between methyl iodide and methyl oxide?arrow_forward
- Consider the four trienes E–H.a.Rank compounds E–H in order of increasing heat of hydrogenation. b.Which compound is most reactive in the Diels–Alder reaction? c. Which compound(s) are unreactive in the Diels–Alder reaction? d.Which compound absorbs the longest wavelength of ultraviolet light?arrow_forwardCan you explain the process of free radical bromination by using methylcyclohexane?arrow_forwardOrganic Chemistry - nucleophilic substitution and elimination rxns chapterarrow_forward
- What alkane is needed to make each alkyl halide by radical halogenation?arrow_forwardWith reference to the indicated C–H bonds in the following compound: a.Rank the C–H bonds in order of increasing bond strength. b.Draw the radical resulting from cleavage of each C–H bond, and classify it as 1°, 2°, or 3°. c. Rank the radicals in order of increasing stability. d.Rank the C–H bonds in order of increasing ease of H abstraction in a radical halogenation reaction.arrow_forwardwrite the reagents and name of rxn plsarrow_forward
- Explain the General Features of Radical Reactions ?arrow_forwardHomolysis of the indicated C–H bond in propene forms a resonancestabilized radical. a.Draw the two possible resonance structures for this radical. b.Use half-headed curved arrows to illustrate how one resonance structure can be converted to the other. c. Draw a structure for the resonance hybrid.arrow_forwardDraw the major organic product for the reaction between 1,2-dimethylcycloheptene and a cold, dilute solution of KMnO4.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY