
(a)
Interpretation:
Enol tautomer of 2,4-pentanedione has to be drawn.
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form. Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.
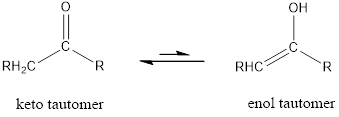
(b)
Interpretation:
Reason for most
Concept Introduction:
Tautomerism is the ability of a molecule to exist in more than one chemical form. Tautomers are formed by the migration of a hydrogen atom, accompanied by the switching of a single and neighboring double bond.
The only difference in keto-enol tautomers is the location of hydrogen and double bond.

Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Essential Organic Chemistry, Global Edition
- These reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4arrow_forwardProvide an appropriate alkyne starting material A and intermediate product B. Omit byproducts. The number of carbon atoms in the starting material should be the same as in the final product. 1. R2BH, THE Alkyne Intermediate Starting Material 2. H2O2, NaOH, H2O A Draw intermediate B. Draw alkyne starting material A. Rings More Erase Select Draw Rings More Erase Select Draw H Harrow_forward5. What reagents are needed to convert toluene (C,H,CH,) to each compound? a. C.H.COOH b. C.H₂CH₂Br c. p-bromotoluene d. o-nitrotoluene e. p-ethyltoluene f.arrow_forward
- b. Explain why alkylation of 2-ethylcyclohexanone at different temperatures results in different products. 1. LDA, -78 °C 2. CHI 1. LDA, -78 °C 2.warm to 0°C 3. CH₂Iarrow_forward4. Which compound is an acetal? O. %3D a. I b. II с. III d. IV e. All of thesearrow_forwardLab 4: Reactions of Aldehydes and Ketones Reactions of Aldehydes and Ketones 2. Which of the following reagents or tests would you use to differentiate between sxiT CH,CH,CH, -Ċ-H and a. Iodoform b. Chromic acid c. Tollens' d. DNP e. More than one answer is correct. Explain your answer:arrow_forward
- Draw a stepwise mechanism for each reaction. Br + CH3CH2NH2 NaOH 'N" + H20 + NaBr a. Br ČH,CH3 NABH4 b. CH,OH + H20 NH2arrow_forwardIdentify the compound that forms the following acetal upon treating with acid and removing water. O A. HO HO. O: OB. HO HO, C. HO, HO HO, OD.arrow_forwardWhat is the reduction product of the following compound with H2/Pd? O. 2-propyl-1-cyclohexanol 1-propyl-2-cyclohexanol 2-propylcyclohexanone O 2-propenyl-1-cyclohexanolarrow_forward
- 6. What starting materials are needed to synthesize each compound using the indicated reagent or functional group? a. Synthesize: OH from an ester b. Synthesize: using an organocuprate reagent 04 using a Grignard reagent c. Synthesize: OHarrow_forwardWhat is the major product of the reaction of 1 mol of propyne with each of the following reagents? a. HBr (1 mol) e. aqueous H2SO4, HgSO4 h. H2/Lindlar catalyst b. HBr (2 mol) f. R2BH in THF followed by i. sodium amide c. Br2 (1 mol)/CH2Cl2 H2O2/HO-/H2O j. the product of part i followed by d. Br2 (2 mol)/CH2Cl2 g. excess H2, Pd/C 1-chloropropanearrow_forward10. What products are formed when the following compound is treated with each reagent? If no reaction occurs, write NR. a. H₂/Pd b. K₂Cr2O7 c. Tollen's reagent d. 2 equiv. CH3OH, H+ e. 2 equiv. CH3CH₂OH, H+ f. Product of (e), then H₂O/H*arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
