
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.23P
What major IR absorptions are present above 1500 cm−1 for each compound?
a. 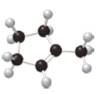 b.
b. 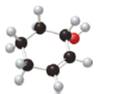
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the systematic name of each organic molecule:
structure
Η
OH OH
OH
OH
H
name
Draw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms, 1 oxygen atom, at least one ring, and no double or triple bonds.
Click and drag to start drawing a
structure.
: ☐
☑
⑤
Name these organic compounds:
structure
name
CH₁₂
CH3 - C
CH
-
CH2
||
CH3-
-
CH₂
CH₂
|
-
-
CH3
CH3
2-methyl-2-butene
☐
3-methyl-1-butyne
-
CH3 CH.
-
C=CH
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - What is the mass of the molecular ion formed from...Ch. 13 - Prob. 13.2PCh. 13 - Use the following information to propose a...Ch. 13 - Prob. 13.4PCh. 13 - What molecular ions would you expect for the...Ch. 13 - The mass spectrum of 2,3-dimethylpentane also...Ch. 13 - The base peak in the mass spectrum of 2, 2,...Ch. 13 - (a) What mass spectral fragments are formed by ...Ch. 13 - What cations are formed in the mass spectrometer...Ch. 13 - The low-resolution mass spectrum of an unknown...
Ch. 13 - Benzene, toluene, and p-xylene BTX are often added...Ch. 13 - Prob. 13.12PCh. 13 - Prob. 13.13PCh. 13 - Prob. 13.14PCh. 13 - Prob. 13.15PCh. 13 - How do the IR spectra of the isomers cyclopentane...Ch. 13 - Problem 13.17 How do the three isomers of...Ch. 13 - Problem 13.18 What functional groups are...Ch. 13 - Problem-13.19 What are the major IR absorptions in...Ch. 13 - Problem-13.20 What are the major IR absorptions in...Ch. 13 - Problem-13.21 Which of the following possible...Ch. 13 - Problem-13.22 Propose structures consistent with...Ch. 13 - 13.23 What major IR absorptions are present above ...Ch. 13 - Problem-13.24 The mass spectrum of the following...Ch. 13 - Prob. 13.25PCh. 13 - Which compound gives a molecular ion at m/z= 122,...Ch. 13 - Propose two molecular formulas for each molecular...Ch. 13 - Propose four possible structures for a hydrocarbon...Ch. 13 - Problem-13.29 What is the molecular formula for...Ch. 13 - Problem-13.30 Propose a molecular formula for rose...Ch. 13 - 13.31 Match each structure to its mass spectrum
Ch. 13 - 13.32 Propose two possible structures for a...Ch. 13 - 13.33 What cations are formed in the mass...Ch. 13 - 13.34 and have the same molecular ion in the...Ch. 13 - 13.35 For each compound, assign likely...Ch. 13 - Prob. 13.36PCh. 13 - 13.37 Propose a structure consistent with each...Ch. 13 - 13.38 A low-resolution mass spectrum of the...Ch. 13 - 13.39 Primary alcohols often show a peak in their...Ch. 13 - 13.40 Like alcohols, ethers undergo α cleavage by...Ch. 13 - 13.41 Which of the highlighted bonds absorbs at...Ch. 13 - 13.42 What major IR absorptions are present above ...Ch. 13 - 13.43 How would each of the following pairs of...Ch. 13 - 13.44 Morphine, heroin, and oxycodone are three...Ch. 13 - 13.45 Reduction of cyclohex-2-enone can yield...Ch. 13 - Prob. 13.46PCh. 13 - 13.47 Match each compound to its IR spectrum
Ch. 13 - 13.48 Propose possible structures consistent with...Ch. 13 - A chiral hydrocarbon X exhibits a molecular ion at...Ch. 13 - 13.50 A chiral compound has a strong absorption...Ch. 13 - 13.51 Treatment of benzoic acid with followed by...Ch. 13 - 13.52 Treatment of benzaldehyde with in aqueous ...Ch. 13 - Prob. 13.53PCh. 13 - 13.54 Reaction of 2-methylpropanoic acid with ...Ch. 13 - 13.55 Reaction of pentanoyl chloride with lithium...Ch. 13 - Prob. 13.56PCh. 13 - 13.57 Treatment of anisole with and forms P,...Ch. 13 - 13.58 Reaction of with forms compound ,...Ch. 13 - Problem-13.59 The carbonyl absorption of an amide...Ch. 13 - Prob. 13.60PCh. 13 - Problem-13.61 Explain why a ketone carbonyl...Ch. 13 - 13.62 Oxidation of citronellol, a constituent of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
Describe Mendels conclusions about how traits are passed from generation to generation.
Concepts of Genetics (12th Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many different molecules are drawn below?arrow_forwardWith the reference to a anion A, Label compounds B-F as an isomer or resonance strcuture of A. FOr each isomer indicate what bonds differs from A. Provide steps and undertanding on how you come up with work.arrow_forwardProvide steps and also tips to undertand how to do on my own. Add the correct number of hydrogen atoms for each carbon atom and lone pairs to each oxygen atom.arrow_forward
- A mixture of oxygen and ethyne is burnt for welding tell why mixture of ethyne and air is not usedarrow_forwardQ2: Draw all applicable resonance forms for the acetate ion CH3COO. Clearly show all lone pairs, charges, and arrow formalism.arrow_forwardIn the following molecule, indicate the hybridization and shape of the indicated atoms. -z: CH3 CH 3 HO: H3C :Ö: CIarrow_forward
- Show mechanism with explanation. don't give Ai generated solutionarrow_forwardPlease Help!!!arrow_forwardQ2: Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor. SO2 NO3 Page 3 of 4 Chem 0310 Organic Chemistry 1 HW Problem Sets CH3NSO (Thionitromethane, skeleton on the right) H N H3C Sarrow_forward
- A 10.00-mL pipet was filled to the mark with distilled water at the lab temperature of 22 oC. The water, delivered to a tared weighing bottle was found to weigh 9.973 g. The density of water at 22 oC is 0.99780 g/mL. Calculate the volume of the pipet in mL. (disregard air displacement for this calculation and record your answer to the proper number of significant digits.)arrow_forwardResonance Formsa) Draw all resonance forms of the molecules. Include curved arrow notation. Label majorresonance contributor.arrow_forwardShow work with explanation needed. Don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY