
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please Help!!!
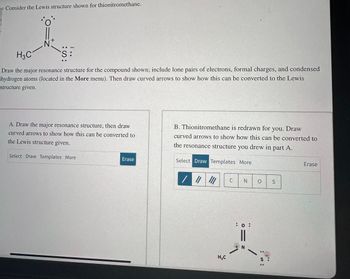
Transcribed Image Text:Consider the Lewis structure shown for thionitromethane.
נתון רב8uI1IP
H3C
N
S:
S:
Draw the major resonance structure for the compound shown; include lone pairs of electrons, formal charges, and condensed
hydrogen atoms (located in the More menu). Then draw curved arrows to show how this can be converted to the Lewis
structure given.
A. Draw the major resonance structure, then draw
curved arrows to show how this can be converted to
the Lewis structure given.
Select Draw Templates More
Erase
B. Thionitromethane is redrawn for you. Draw
curved arrows to show how this can be converted to
the resonance structure you drew in part A.
Select Draw Templates More
Erase
// W
C
N
0
S
: 0 :
H₂C
S
: 5:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Draw a resonance structure of the compound shown below, called 2-heptanone, which is found in some kinds of cheese. en Draw curved arrow(s) on the initial structure to show the expected resonance. Modify the second structure given to draw a new resonance structure, including any relevant formal charges. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H3C CH3 Edit Drawing H3C CH3arrow_forwarda Draw the additional resonance structure(s) of the structure below? :CH₂-C=N: • Include all valence lone pairs in your answer. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the → symbol from the drop-down menu. Ne **** H₂C=C=N: ChemDoodleⓇ On [F removearrow_forwardOCHEM HELP!!! Provide ALL the resonance structures for the compounds shown below (see the attached picture). Also draw ALL the valence electrons (lone pairs) on the given compound and the resonance structures drawn and use the correct arrows between the resonance structures drawnarrow_forward
- Draw the best Lewis structure for HCN and determine the formal charge on the nitrogen. Please just put in single number for answer. If it is positive no need for the +, if it is negative, please use the -.arrow_forwardNEED A HELP WITH THESEarrow_forwardIn the following Lewis structure of [BrO3]", every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). Ö: Br: 2+ 2-arrow_forward
- Draw resonance structures for the following compound: Add curved arrow(s) to show resonance using one of the five patterns, and modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H₂C CH3 N. H3C CH3 Edit Drawingarrow_forwardThe following line angle drawings have all lone pairs shown. Add formal charges on specific atoms wherever they are needed to complete the structures. Hint: both molecules are overall neutral! :0 :0: H. : :0:arrow_forwardWhich of the following species is a valid resonance structure of A? Usecurved arrows to show how A is converted to any valid resonancestructure. When a compound is not a valid resonance structure of A,explain why not.arrow_forward
- Draw resonance structures for the following compound: Add curved arrow(s) to show resonance using one of the five patterns, and modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H3C. CH3 x H3C CH₂ Edit Drawing H3C H3C. + CH 3 CH3arrow_forwardN°H HN. HO но. HN the lone pairs to help you track the movement of electrons. Note that all the charges are as shown but the lone pairs of electrons might be omitted so, add 6. Using curved arrows draw at least one resonance structure for each of the following species.arrow_forwardThree major contributing resonance structures are possible for the following cation. One is given below. Draw the remaining structures (in any order), including nonbonding electrons and formal charges. Omit curved arrows. н н CH3З Which contributes most to the hybrid? The structure with the positive charge on sulfur. All contribute equally. O The structures with the positive charge on carbon.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning