
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.21P
Which of the following possible structures for X can be excluded on the basis of its IR spectrum?
a.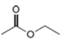 b.
b.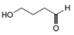 c.
c.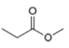 d.
d.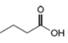
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following possible structures for X can be excluded on the basis of its IR spectrum?
A
B.
C.
D.
но
H3C
но
This is most likely to be the IR spectrum of:
00 0
DOST
000
D.
Answer the following questions:
a. What is the relationship between chemical shift in ppm and operating frequency? b. What is the relationship between chemical shift in hertz and operating frequency? c. What is the relationship between coupling constant in hertz and operating frequency? d. How does the operating frequency in NMR spectroscopy compare with the operating frequency in IR and UV/Vis spectroscopy?
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - What is the mass of the molecular ion formed from...Ch. 13 - Prob. 13.2PCh. 13 - Use the following information to propose a...Ch. 13 - Prob. 13.4PCh. 13 - What molecular ions would you expect for the...Ch. 13 - The mass spectrum of 2,3-dimethylpentane also...Ch. 13 - The base peak in the mass spectrum of 2, 2,...Ch. 13 - (a) What mass spectral fragments are formed by ...Ch. 13 - What cations are formed in the mass spectrometer...Ch. 13 - The low-resolution mass spectrum of an unknown...
Ch. 13 - Benzene, toluene, and p-xylene BTX are often added...Ch. 13 - Prob. 13.12PCh. 13 - Prob. 13.13PCh. 13 - Prob. 13.14PCh. 13 - Prob. 13.15PCh. 13 - How do the IR spectra of the isomers cyclopentane...Ch. 13 - Problem 13.17 How do the three isomers of...Ch. 13 - Problem 13.18 What functional groups are...Ch. 13 - Problem-13.19 What are the major IR absorptions in...Ch. 13 - Problem-13.20 What are the major IR absorptions in...Ch. 13 - Problem-13.21 Which of the following possible...Ch. 13 - Problem-13.22 Propose structures consistent with...Ch. 13 - 13.23 What major IR absorptions are present above ...Ch. 13 - Problem-13.24 The mass spectrum of the following...Ch. 13 - Prob. 13.25PCh. 13 - Which compound gives a molecular ion at m/z= 122,...Ch. 13 - Propose two molecular formulas for each molecular...Ch. 13 - Propose four possible structures for a hydrocarbon...Ch. 13 - Problem-13.29 What is the molecular formula for...Ch. 13 - Problem-13.30 Propose a molecular formula for rose...Ch. 13 - 13.31 Match each structure to its mass spectrum
Ch. 13 - 13.32 Propose two possible structures for a...Ch. 13 - 13.33 What cations are formed in the mass...Ch. 13 - 13.34 and have the same molecular ion in the...Ch. 13 - 13.35 For each compound, assign likely...Ch. 13 - Prob. 13.36PCh. 13 - 13.37 Propose a structure consistent with each...Ch. 13 - 13.38 A low-resolution mass spectrum of the...Ch. 13 - 13.39 Primary alcohols often show a peak in their...Ch. 13 - 13.40 Like alcohols, ethers undergo α cleavage by...Ch. 13 - 13.41 Which of the highlighted bonds absorbs at...Ch. 13 - 13.42 What major IR absorptions are present above ...Ch. 13 - 13.43 How would each of the following pairs of...Ch. 13 - 13.44 Morphine, heroin, and oxycodone are three...Ch. 13 - 13.45 Reduction of cyclohex-2-enone can yield...Ch. 13 - Prob. 13.46PCh. 13 - 13.47 Match each compound to its IR spectrum
Ch. 13 - 13.48 Propose possible structures consistent with...Ch. 13 - A chiral hydrocarbon X exhibits a molecular ion at...Ch. 13 - 13.50 A chiral compound has a strong absorption...Ch. 13 - 13.51 Treatment of benzoic acid with followed by...Ch. 13 - 13.52 Treatment of benzaldehyde with in aqueous ...Ch. 13 - Prob. 13.53PCh. 13 - 13.54 Reaction of 2-methylpropanoic acid with ...Ch. 13 - 13.55 Reaction of pentanoyl chloride with lithium...Ch. 13 - Prob. 13.56PCh. 13 - 13.57 Treatment of anisole with and forms P,...Ch. 13 - 13.58 Reaction of with forms compound ,...Ch. 13 - Problem-13.59 The carbonyl absorption of an amide...Ch. 13 - Prob. 13.60PCh. 13 - Problem-13.61 Explain why a ketone carbonyl...Ch. 13 - 13.62 Oxidation of citronellol, a constituent of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C. Which FTIR spectra correspond to (a) PVAc and (b) PVA? Justify your answer. D. Poly(vinyl butyral), or PVB, is a thermoplastic polymer used as an interlayer in laminated glass, paints, lacquers, varnishes, and adhesives. The structure of PVB is shown below. Show how you will prepare PVB from PVA.arrow_forward4) Which two pairs cannot be distinguished by using IR spectroscopy? a. b. d e. all of them could be distinguishedarrow_forwardQ.No.2 What is spectroscopy?. What types of analysis you can do with this technique ?. Write a common procedure that you can adapt when you will perform any analysis with Spectroscopy.arrow_forward
- MATCH a structure from the list below to the following IR spectra. HO D. A. OH В. E. H. C. F. 50 4D00 3000 2000 1000 SB0 TRENSHETTENCEIarrow_forwardHow does the operating frequency in NMR spectroscopy compare with the operating frequency in IR and UV/Vis spectroscopy?arrow_forwardPlease answer in typewritten and essay form. Thank you. Brief explanation only (2-3 sentences each factors) How do the following factors affect absorption frequencies? A. O-H vs O-D. B. CH stretch, hybrid orbitals used by carbonarrow_forward
- Atomic absorption spectroscopy results are highly reproducible but have low sensitivity and efficiency. This is due to which of the following? A. B. The high temperatures of analysis often destroy the atoms B. C. The amount of dilution due to mixing with large volumes of combustion gases C. A. The small amount of analyte that actually reaches the flame D. A and C E. A and Barrow_forwardHow do the following factors affect absorption frequencies? A. O-H vs O-D. B. CH stretch, hybrid orbitals used by carbon Please answer it in typewritten thank youarrow_forwardMorphine, heroin, and oxycodone are three addicting analgesic narcotics. How could IR spectroscopy be used to distinguish these three compounds from each other?arrow_forward
- HOW nmr spectroscopy is used in medical instrument ?arrow_forward1. What is the number of signals that should be observed in the ¹H NMR spectra of each of these molecules? a. b. C. OH H₂N Text Textarrow_forwardB.19 What major IR absorptions are present above 1500 cm ¹ for each compound? a. b. C. d. OH OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY