
Interpretation:
Structure of ciprofloxacin hydrochloride has to be drawn and the
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
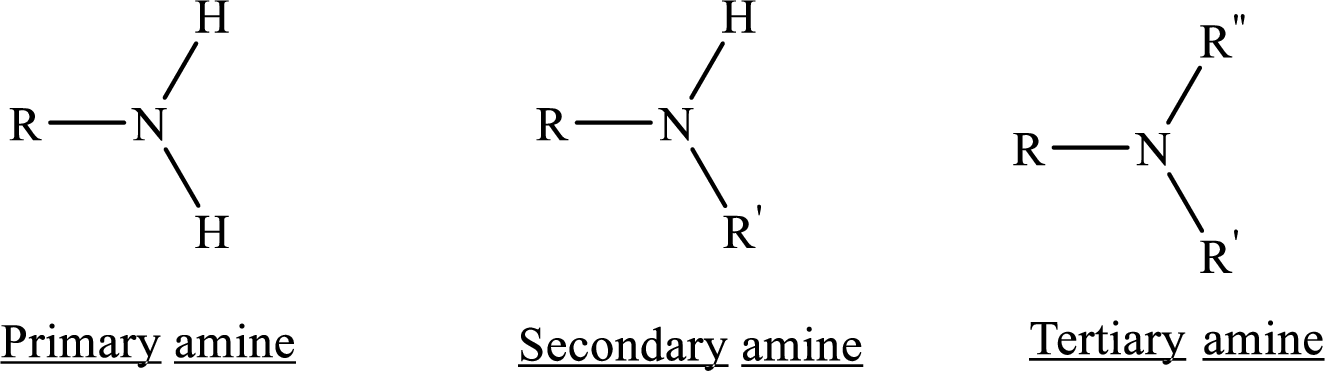
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- Which of the compounds, ethanol, C2H6O, or methanol, CH3OH, should have the higher boiling point? Why? How would the boiling point change if the atmospheric pressure increased or decreased? What is the effect of small amount of impurity on the boiling point of an organic compound? List five physical properties of organic compounds that are often measured by organic chemists.arrow_forwardwhat are the functional groups in the molecule C3H8O?arrow_forwardName 5 common organic compounds that are found in your home. Draw their Lewis structures and give their molecular formula. Encircle and identify the functional group/s that is/ are present in each molecule. Write your answers in another sheet of paper.arrow_forward
- 1. MgBr (xs), CH3CH₂OCH₂CH3 2. HCI, H₂Oarrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forward"Cyclohexene is a linear molecule with 6 carbon Describe the molecule cyclohexene and provide the chemical formula. atoms and 12 hydrogen atoms. There is a triple bond because it ends in - # 4 (Glycerina) ene. The chemical formula is C6H12" Which two functional groups are in the molecule shown below? Use the terms left and right to distinguish them. "The left functional group is a carboxylic acid. The right functional group is an #5 (Trinitress) alcohol." Harrow_forward
- The clear formula of an active drug substance is given below. Write the closed formula of this compound, it consists of the combination of two organic compounds that we have seen so far. Write the name of these two structures.arrow_forward1. There are five structural isomers for hexane, C6H₁4. Draw the five structures using simplified structural formulae. For example, one of the isomers of butane, C4H₁0, could be drawn as CH3CH₂CH₂CH3. Use the same sort of format for the other questions on this page. 2. STRUCTURAL ISOMERISM 3. Draw as many structural isomers as possible for C3H8O. Draw as many structural isomers as you can for CH₂0 containing a benzene ring. 4. Draw as many structural isomers as you can for C4H8O₂ containing the grouparrow_forwardThe chemical formula C4H10O results in four alcohols and three ethers for a total of seven structuralisomers. Draw pairs of structural formulas for these molecules that illustrate positional and functional isomerism on a sheet of paper. You will be drawing a total of four formulas. Label each pair as positional or functional.arrow_forward
- Break open your cyclohexane ring to make n-hexane (C6H14). (You will need two extra hydrogen atoms to the terminal carbon atoms to complete your model). Is hexane flexible or rigid? The melting point of cyclohexane is 6o C whereas the melting point of hexane is -94o C. Can you suggest a reason for this based on the structures you have made?arrow_forwardLook up the structures of vitamin C and vitamin E on the Web, and identify the functional groups in these vitamins.arrow_forward8. Write the structural formulas of both isomers with the formula C2H6O. Label the functional group of each isomer.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





