
Concept explainers
(a)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
(b)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Alcohol: It is an organic compound where it contains at least one
(c)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
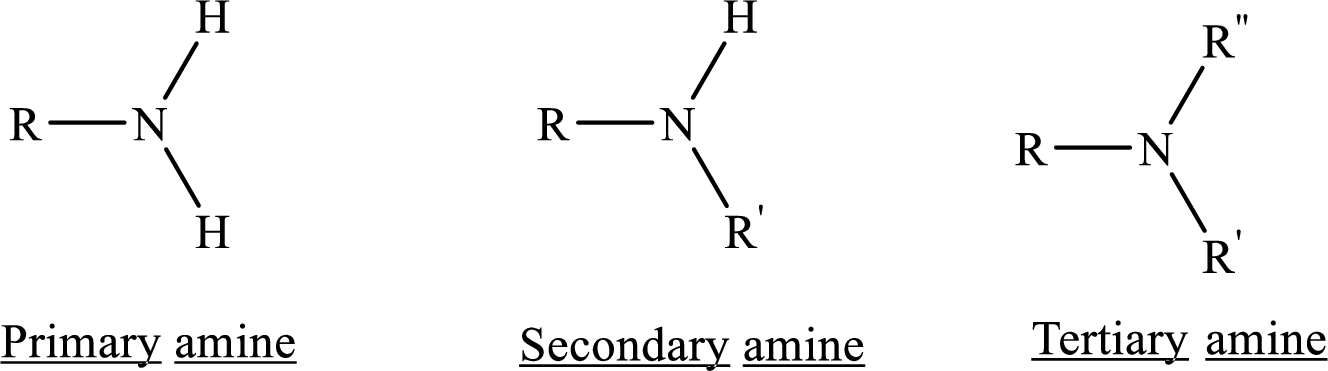
(d)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Ester: One
(e)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- ONLY QUESTION 7 AND 8 ANSWER PLEASE Experiment 3: C4H8 1. Attach 2 C atoms in a straight line. Put the double bond between C1 and C2. 2. Attach 2 more C atoms so that the C1C2C3 bond angle is about 120° and the C2C3C4 bond angle is about 109°. 3. Attach toothpicks to indicate the bonds to H atoms to complete the structure. Question 6 Take a photo and label it P6. Name the structure. Does this structure have any geometric isomers? If so draw and name them. If not, explain why there are no geometric isomers? 4. Now move the double bond so that it is between C2 and C3. Adjust other CCC bond angles appropriately. Question 7 Take a photo and label it P7. Name the structure. Does this structure have any geometric isomers? If so draw and name them. If not, explain why there are no geometric isomers? Question 8 Do you need to move the double bond to between C3 and C4 to check for more isomers? Why or why not?arrow_forwardprob set 1 polar molecules topicarrow_forwardQuestion: Experiment 2: C3H6 1. Attach 2 C atoms with a double bond between C1 and C2. 2. Attach a 3rd C atom to C2 so that the C1C2C3 bond angle is 120°. 3. Then attach toothpicks representing the C-H bonds to complete the structure. Question 3 Draw the structure and name the compound. Take a photo and label it P3. Question 4 Identify the groups on C1. Are they the same or different? Identify the groups on C2. Are they the same or different? Will there be cis and trans isomers, explain your answer? Question 5 Is there any other way to arrange 3 C atoms and 6 H atoms? Ignore cyclic compounds. If so, identify any geometric isomers.arrow_forward
- ( plz do not copy give correct answer )arrow_forwardHow can I solve this question? I need full detail and accurate answer.arrow_forwardCircle the letter of the correct answer. 1. Which of the following compounds is saturated? (a) propene (b) octane (c) 3-heptyne (d) 2-butene (e) 1-butene 2. Which formula represents an alkyne? (a) C5H8(b) C5H12(c) C5H16(d) C5H14(e) C5H10 3. What is the total number of covalent bonds in a molecule of C4H10O? (a) 10 (b) 12 (c) 14 (d) 16 (e) 18 4. What is the name of the alkyl group containing three carbon atoms? (a) diphenyl (b) trimethyl (c) propyl (d) methyl (e) butyl 5. What is the IUPAC name of the following molecule? (a) 2-bromo-4-isopropyl-3-methylbutane (b) 2-bromo-3,5-dimethylhexane (c) 3,4-dimethyl-2-bromohexane (d) 2-bromo-3-methyl-4-propylbutane (e) 2-bromo-3,5-dimethylheptane 6. Which of the following compounds is considered to be non-polar? (a) CH3CH2OH (b) CH3CHO (c) CH3COOH (d) CH3CH2NH2(e) CH3CH2CH3 (e) Ethylene glycol is insoluble in water. 7. The molecule CH3COCH3 is classified as: (a) an ether (b) an ester (c) an aldehyde (d) a ketone (e) a carboxylic acid 8.…arrow_forward
- Please help me solve problem. TY!arrow_forwardFor both molecules below, draw the most appropriate Lewis structure, include any non-zero formal charges on the appropriate atoms, name the electron group geometry, name the molecular geometry, and redraw the molecule to show the molecular geometry, labelling the bond angles. a. SiH2Cl2 b. XeH2F2 Please Fast Answer.arrow_forwardHi! Can you help me figure out if the following 7 compounds are chiral or not? I understand that we need to consider plane of symmetry and center of symmetry, but I'm not too sure of my answers while doing these practice problems.arrow_forward
- Can you help me find all of the electron geometry tetrahedral bonds in this Vitimine A structure? The photo attached is one I got wrong. Thank you.arrow_forwardHey help me please! These are only tree question that I am able to ask on the app so please answer all of them it’s important thank you so much!arrow_forwardGive typed explanation What is the missing reactant in this organic reaction? CH3 OH I CH3-CH-CH-CH3 R+ H₂ Pt Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. C с Xarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
