
Chemistry In Context
9th Edition
ISBN: 9781259638145
Author: Fahlman, Bradley D., Purvis-roberts, Kathleen, Kirk, John S., Bentley, Anne K., Daubenmire, Patrick L., ELLIS, Jamie P., Mury, Michael T., American Chemical Society
Publisher: Mcgraw-hill Education,
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 12.7YT
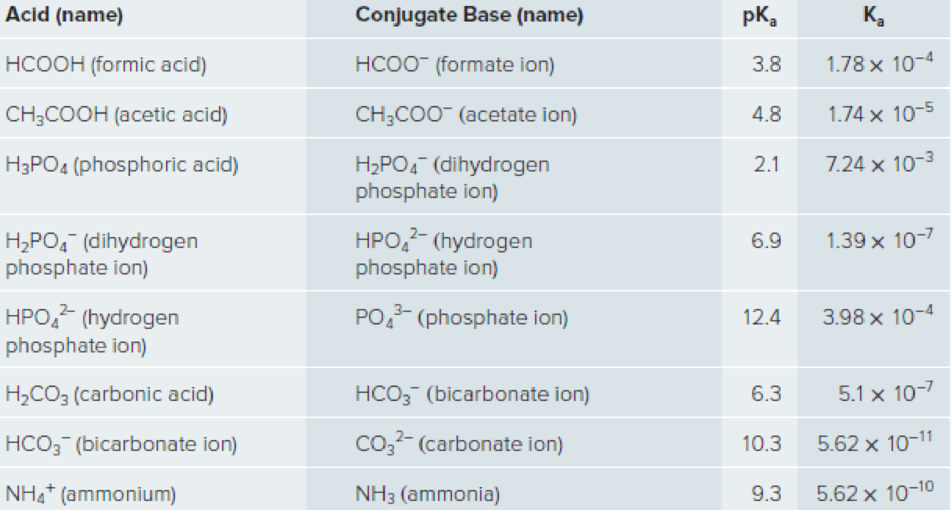
A practicing scientist must Judge a potential buffer for its utility in mimicking different cellular environments. Consider the list of target pH values and match them to the best possible chemical mixture for creating that pH, see Table 12.1 for a list.
- a. pH 2.5
- b. pH 7.4
- c. pH 6.8
- d. pH 5.4
Table 12.1 Dissociation of Some Weak Acids with Ka and pKa Values
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.
Draw a Haworth projection or a common cyclic form of this monosaccharide:
H-
-OH
H-
OH
H-
-OH
CH₂OH
Answer the question in the first photo
Chapter 12 Solutions
Chemistry In Context
Ch. 12.1 - Skill Building Finding Equilibrium Glucose and...Ch. 12.1 - Prob. 12.3YTCh. 12.1 - Prob. 12.4YTCh. 12.2 - Prob. 12.5YTCh. 12.2 - Prob. 12.6YTCh. 12.2 - A practicing scientist must Judge a potential...Ch. 12.3 - Prob. 12.8YTCh. 12.3 - Skill Building Checking on Carbon a. Examine the...Ch. 12.3 - Prob. 12.10YTCh. 12.3 - Prob. 12.11YT
Ch. 12.3 - Prob. 12.12YTCh. 12.4 - Prob. 12.13YTCh. 12.4 - Skill Building Functional Groups in Dopamine Draw...Ch. 12.4 - Prob. 12.15YTCh. 12.5 - Prob. 12.16YTCh. 12.5 - Prob. 12.17YTCh. 12.6 - Prob. 12.18YTCh. 12.6 - Prob. 12.19YTCh. 12.6 - The structures of proteins, such as the ones shown...Ch. 12.7 - Reconsider your work in past chapters. List three...Ch. 12.7 - Prob. 12.22YTCh. 12.7 - Prob. 12.23YTCh. 12.8 - Prob. 12.24YTCh. 12.8 - Prob. 12.25YTCh. 12.9 - Skill Building Ester Formation Draw structural...Ch. 12.9 - Prob. 12.27YTCh. 12.9 - You Decide Supersize My Aspirin A friend who...Ch. 12.9 - Modern methods of drug discovery involve...Ch. 12.10 - Make two lists of drugs for each of the two...Ch. 12.10 - See for yourself the shapes of drug molecules by...Ch. 12.10 - Prob. 12.33YTCh. 12.10 - Prob. 12.34YTCh. 12 - Scientific Practices Follow the Hormone Using the...Ch. 12 - The field of chemistry has many sub-disciplines....Ch. 12 - Prob. 2QCh. 12 - Prob. 4QCh. 12 - Nitrous acid (HNO2) has a Ka value of 4.0 10 4,...Ch. 12 - Use the Henderson-Hasselbalch equation and Table...Ch. 12 - Write the structural formula and line-angle...Ch. 12 - Prob. 8QCh. 12 - Prob. 9QCh. 12 - Prob. 10QCh. 12 - Prob. 11QCh. 12 - Prob. 12QCh. 12 - Estradiol is relatively insoluble in water but...Ch. 12 - Prob. 14QCh. 12 - Prob. 15QCh. 12 - Prob. 16QCh. 12 - Define and relate the two terms: hormone and...Ch. 12 - Refer to Figure 11.17. Select two examples of...Ch. 12 - Prob. 19QCh. 12 - Molecules as diverse as cholesterol, sex hormones,...Ch. 12 - Prob. 21QCh. 12 - Prob. 22QCh. 12 - Prob. 23QCh. 12 - Sulfanilamide is the simplest sulfa drug, a type...Ch. 12 - Explain why an equilibrium constant cannot tell...Ch. 12 - Use the information in Table 12.1 to redraw Figure...Ch. 12 - Draw structural formulas for each of these...Ch. 12 - In Your Turn 12.12, you were asked to draw...Ch. 12 - Prob. 29QCh. 12 - Prob. 30QCh. 12 - Prob. 31QCh. 12 - Prob. 32QCh. 12 - Prob. 34QCh. 12 - Prob. 35QCh. 12 - Prob. 36QCh. 12 - Prob. 37QCh. 12 - Prob. 38QCh. 12 - The text states that some racemic mixtures contain...Ch. 12 - Prob. 40QCh. 12 - Prob. 41QCh. 12 - Prob. 44QCh. 12 - Prob. 47QCh. 12 - Prob. 49QCh. 12 - Dorothy Crowfoot Hodgkin first determined the...Ch. 12 - Prob. 52Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward
- Is nucleophilic acyl substitution an SN1 or SN2 reaction?arrow_forwardDraw product A, indicating what type of reaction occurs. NH2 F3C CF3 NH OMe NH2-NH2, ACOH Aarrow_forwardPhotochemical smog is formed in part by the action of light on nitrogen dioxide. The wavelength of radiation absorbed by NO2 in this reaction is 197 nm.(a) Draw the Lewis structure of NO2 and sketch its π molecular orbitals.(b) When 1.56 mJ of energy is absorbed by 3.0 L of air at 20 °C and 0.91 atm, all the NO2 molecules in this sample dissociate by the reaction shown. Assume that each absorbed photon leads to the dissociation (into NO and O) of one NO2 molecule. What is the proportion, in parts per million, of NO2 molecules in this sample? Assume that the sample behaves ideally.arrow_forward
- Correct each molecule in the drawing area below so that it has the skeletal ("line") structure it would have if it were dissolved in a 0.1 M aqueous solution of HCI. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO Explanation Check NH, 2 W O :□ G ©2025 M unter Accessibilityarrow_forwardAn expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.arrow_forwardThe reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?arrow_forward
- One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY