
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 12, Problem 44P
Interpretation Introduction
Interpretation:
The value for the relative east of removing a hydrogen atom from tertiary, secondary and primary hydrogen carbons by a chlorine radical has to be given.
Concept introduction:
Radical or free radical: unpaired valence electron of an atom, molecule, or ion is called as radical.
Chlorine radical forms a tertiary radical five times faster than a primary radical and it forms a secondary radical 3.8 times faster than a primary radical.
Relative rates of alkyl radical formation by a chlorine radical at 125 °C.
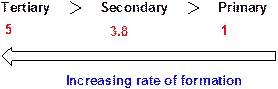
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A chemist wanted to determine experimentally the relative ease of removing a hydrogen atom from a tertiary, a secondary, and a primary carbon by a chlorine radical. He allowed 2-methylbutane to undergo chlorination at 300 °C and obtained as products 36% 1-chloro-2-methylbutane, 18% 2-chloro-2-methylbutane, 28% 2-chloro-3-methylbutane, and 18% 1-chloro-3-methylbutane. What values did he obtain for the relative ease of removing a hydrogen atom from tertiary, secondary, and primary hydrogen carbons by a chlorine radical under the conditions of his experiment?
Radical Mono-Chlorination of pentane is a poor way to prepare 1-chloropentane, but radical
chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride. Write an
equation for both of these reactions and explain why this is.
Pentane
Neopentane
2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.
Chapter 12 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 12.2 - Prob. 1PCh. 12.2 - Write the steps for formation of...Ch. 12.3 - Prob. 3PCh. 12.4 - How many alkyl chlorides are obtained from...Ch. 12.4 - Prob. 6PCh. 12.5 - Prob. 8PCh. 12.5 - a. Would chlorination or bromination produce a...Ch. 12.5 - Show how the following compounds could be prepared...Ch. 12.6 - Prob. 12PCh. 12.7 - Prob. 13P
Ch. 12.7 - Prob. 14PCh. 12.8 - Prob. 15PCh. 12.8 - Draw the stereoisomers of the major...Ch. 12.9 - Prob. 18PCh. 12.9 - How many allylic substituted bromoalkenes are...Ch. 12.9 - a. How many stereoisomers are formed from the...Ch. 12.9 - Prob. 21PCh. 12.9 - Prob. 22PCh. 12.10 - Prob. 23PCh. 12.11 - How many atoms share the unpaired electrons in...Ch. 12.11 - Prob. 25PCh. 12 - Prob. 26PCh. 12 - Prob. 27PCh. 12 - Prob. 28PCh. 12 - Prob. 29PCh. 12 - Prob. 30PCh. 12 - Prob. 31PCh. 12 - Prob. 32PCh. 12 - Prob. 33PCh. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - a. What five-carbon alkene forms the same product...Ch. 12 - Prob. 39PCh. 12 - Starting with cyclohexane, how could the following...Ch. 12 - a. Propose a mechanism for the following reaction:...Ch. 12 - What stereoisomers are obtained from the following...Ch. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Draw the products of the following reactions,...Ch. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Explain why the rate of bromination of methane...Ch. 12 - Prob. 51PCh. 12 - Prob. 1PCh. 12 - Prob. 2PCh. 12 - Prob. 3PCh. 12 - Prob. 4P
Knowledge Booster
Similar questions
- When 2-methylpropane is monochlorinated in the presence of light at room temperature, 36% of the product is 2-chloro-2-methylpropane and 64% is 1-chloro-2-methylpropane. From these data, calculate how much easier it is to remove a hydrogen atom from a tertiary carbon than from a primary carbon under these conditions.arrow_forwardFree‑radical halogenation can occur with chlorine and a source of direct UV radiation or sunlight. Chlorination of 2,4‑dimethylpentane via radical halogenation leads to the formation of all three of the products shown. Estimate the relative percentages of each product that will be formed using this means of halogenation. Presume that 1 equivalent of chlorine is used.arrow_forwardThe compound below is treated with chlorine in the presence of light. CH3 CH3 CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Sn [F ?arrow_forward
- Define the Mechanism of the Radical Addition of HBr to an Alkene ?arrow_forwardThe chlorination of ethane produces many by-products. One by-product that forms in small amounts is 2-chlorobutane. Suggest a mechanism for the formation of 2-chlorobutane from the radical chlorination of ethane. (NOTES: intermediates can only have one radical, 2-chlorobutane should be the product of your termination step, any intermediate radical formed during the propagation phase can be used in the termination step)arrow_forwardPhotochemical chlorination of 2,2,4-trimethylpentane gives four isomeric monochlorides. (a) Write structural formulas for these four isomers. (b) The two primary chlorides make up 65% of the monochloride fraction. Assuming that all the primary hydrogens in 2,2,4-trimethylpentane are equally reactive, estimate the percentage of each of the two primary chlorides in the product mixture.arrow_forward
- Alkane chlorination can occur at any position in the alkane chain. Draw and name all monochloro products you might obtain from radical chlorination of 3-methylpentane.arrow_forwardThe compound below is treated with chlorine in the presence of light. CH3 CH3CHCH₂CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. n [ ]# ?arrow_forwardWrite the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethanearrow_forward
- Ethers can be prepared by reaction of an alkoxide or phenoxide ion with a primary alkyl halide. Draw the structure of the expected organic product of the reaction of iodomethane with the following alkoxide ion: CH3 H3C O Na You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Do not include counter-ions, e.g., Na", I, in your answer. орy вste ChemDoodlearrow_forwardIn radical chlorination of alkanes, non-equivalent hydrogens react with chlorine atoms at different rates. At 35 °C, primary, secondary, and tertiary C-H bonds react at relative rates of 1: 3.9 : 5.2 respectively. These are conditions of kinetic control where product ratios are determined by relative rates of formation. For example, if A is formed twice as fast as B, the A:B product ratio will be 2. Consider chlorination of the alkane below at 35 °C. 1. Specify the most reactive C-H bond(s), a-c. Two non-equivalent C-H bonds of comparable reactivity should be separated by commas, i.e. a,c. 2. Specify the site of chlorination in the major monochloro substitution product, а-с. Two products that form in comparable quantities should be separated by commas, i.e. a,carrow_forwardIn radical chlorination of alkanes, non-equivalent hydrogens react with chlorine atoms at different rates. At 35 °C, primary, secondary, and tertiary C-H bonds react at relative rates of 1 : 3.9: 5.2 respectively. These are conditions of kinetic control where product ratios are determined by relative rates of formation. For example, if A is formed twice as fast as B, the A:B product ratio will be 2. Consider chlorination of the alkane below at 35 °C. 1. Specify the most reactive C-H bond(s), a-c. Two non-equivalent C-H bonds of comparable reactivity should be separated by commas, 1.e. a,c. 2. Specify the site of chlorination in the major monochloro substitution product, a-c. Two products that form in comparable quantities should be separated by commas, I.e. a,carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning