
Concept explainers
(a)
Interpretation:
Using necessary organic or inorganic reagents, the synthesis of given compounds has to be indicated.
Concept introduction:
Anti-Markovnikov’s rule: Unsymmetrical
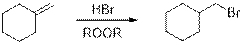
(b)
Using necessary organic or inorganic reagents, the synthesis of given compounds has to be indicated.
Concept introduction:
Anti-Markovnikov’s rule: Unsymmetrical alkene reacts with hydrogen halide, halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
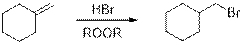
(c)
Interpretation:
Using necessary organic or inorganic reagents, the synthesis of given compounds has to be indicated.
Concept introduction:
Anti-Markovnikov’s rule: Unsymmetrical alkene reacts with hydrogen halide, halide ions goes to the less substitution position of carbon-carbon double bond which provides alkyl halides.
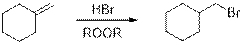
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid and gives alkene.
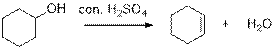
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
(d)
Interpretation:
Using necessary organic or inorganic reagents, the synthesis of given compounds has to be indicated.
Concept introduction:
Alkenes undergoes bromination reaction using bromine which yields the dibromo compound, bromination takes place in the double bond of the alkenes.
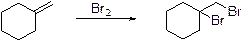
Heck reaction:
The Heck reaction is a cross-coupling reaction of an alkyl halide with an alkene to make a substituted alkenes using palladium as a catalyst and a base.
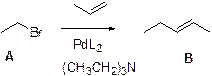
Hydrogenation:
Alkenes undergoes hydrogenation using Pd/C and hydrogen will reduce alkene to
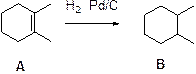
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield? c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?arrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forward
- The ketone 2-heptanone has been identified as contributing to the odor of a number of dairy products, including condensed milk and cheddar cheese. Describe the synthesis of 2-heptanone from acetylene and any necessary organic and inorganic reagents.arrow_forwardFollowing is the structural formula of the tranquilizer meparfynol (Oblivon). Propose a synthesis for this compound starting with acetylene and a ketone. (Notice the -yn- and -ol in the chemical name of this compound, indicating that it contains alkyne and hydroxyl functional groups.)arrow_forwardSeveral diamines are building blocks for the synthesis of pharmaceuticals and agro-chemicals. Show how both 1,3-propanediamine and 1,4-butanediamine can be prepared from acrylonitrile.arrow_forward
- Alcohols undergo dehydration reactions in the presence of an acid catalyst. Which of the following compounds yields only a single alkene product upon dehydration?arrow_forwardProvide the reagents for the reactions.arrow_forwardGive the main organic product for the following reaction: CH2OH OH H3O* CH3CH2CH2OH OH OHarrow_forward
- Give IUPAC names for the following aromatic compounds:arrow_forward3)The syntheses given below are required starting from the starting compounds organic and synthesize using inorganic reagents a) ethyl acetylene ÓH он b) Pharrow_forwardfor the following compounds, give their IUPAC and common names:arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

