
(a)
Interpretation:
The sketch of galvanic cell along with cathode and anode and the direction of electron flow, the direction of flow of ions through salt bridge, the balanced chemical equation and the E0cell should be predicted.
Concept Introduction:
In galvanic cell chemical energy converted into electrical energy.
At anode oxidation takes place which means loss of electrons.
At cathode reduction takes place which means gain of electrons.
(a)
Answer to Problem 21E
The E0cell is0.27 V
Reaction at anode:
Reaction at cathode:
Overall reaction:
Explanation of Solution
Given information:
The diagram of cell is shown below:
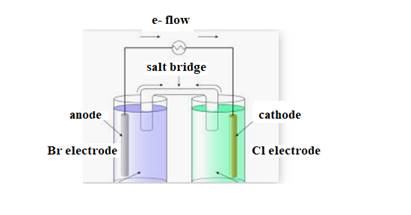
The direction of flow of electrons is from anode to cathode.
Negative ions flow towards anode.
At anode oxidation takes place
At cathode reduction takes place.
The cell representation is shown below:
The oxidation half-cell reaction is shown below:
The reduction half-cell reaction is shown below:
The overall reaction is shown below:
The calculation of E0cell is shown below:
E0cell = E0cathode − E0anode
= 1.36 − (-1.09)
= 0.27 V
(b)
Interpretation:
The sketch of galvanic cell along with cathode and anode and the direction of electron flow, the direction of flow of ions through salt bridge, the balanced chemical equation and the E0cell should be predicted.
Concept Introduction:
In galvanic cell chemical energy converted into electrical energy.
At anode oxidation takes place which means loss of electrons.
At cathode reduction takes place which means gain of electrons.
(b)
Answer to Problem 21E
The E0cell is 0.09 V
Reaction at anode:
Reaction at cathode:
Overall reaction:
Explanation of Solution
Given information:
The diagram is shown below:
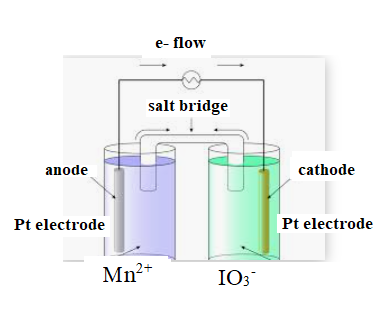
The direction of flow of electrons is from anode to cathode.
Negative ions flow towards anode.
At anode oxidation takes place
At cathode reduction takes place.
The oxidation half-cell reaction is shown below:
The reduction half-cell reaction is shown below:
The overall reaction is shown below:
The calculation of E0cell is shown below:
E0cell = E0cathode − E0anode
= 1.60 − 1.51
= 0.09 V
(c)
Interpretation:
The sketch of galvanic cell along with cathode and anode and the direction of electron flow, the direction of flow of ions through salt bridge, the balanced chemical equation and the E0cell should be predicted.
Concept Introduction:
In galvanic cell chemical energy converted into electrical energy.
At anode oxidation takes place which means loss of electrons.
At cathode reduction takes place which means gain of electrons.
(c)
Answer to Problem 21E
The E0cell is 1.10 V
Reaction at anode:
Reaction at cathode:
Overall reaction:
Explanation of Solution
Given information:
The diagram is shown below:
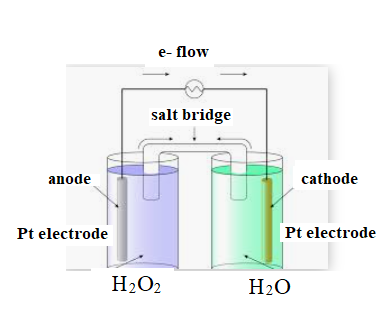
The direction of flow of electrons is from anode to cathode.
Negative ions flow towards anode.
At anode oxidation takes place
At cathode reduction takes place.
The oxidation half-cell reaction is shown below:
The reduction half-cell reaction is shown below:
The overall reaction is shown below:
The calculation of E0cell is shown below:
E0cell = E0cathode − E0anode
= 1.78 − 0.68
= 1.10 V
(d)
Interpretation:
The sketch of galvanic cell along with cathode and anode and the direction of electron flow, the direction of flow of ions through salt bridge, the balanced chemical equation and the E0cell should be predicted.
Concept Introduction:
In galvanic cell chemical energy converted into electrical energy.
At anode oxidation takes place which means loss of electrons.
At cathode reduction takes place which means gain of electrons.
(d)
Answer to Problem 21E
The E0cell is 1.14 V
Reaction at anode:
Reaction at cathode:
Overall reaction:
Explanation of Solution
Given information:
The diagram is shown below:
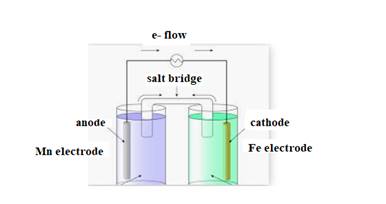
The direction of flow of electrons is from anode to cathode.
Negative ions flow towards anode.
At anode oxidation takes place
At cathode reduction takes place.
The oxidation half-cell reaction is shown below:
The reduction half-cell reaction is shown below:
The overall reaction is shown below:
The calculation of E0cell is shown below:
E0cell = E0cathode − E0anode
= - 0.036- (-1.18)
= 1.14 V
Want to see more full solutions like this?
Chapter 11 Solutions
Chemical Principles
- A potassium chloride solution is electrolyzed by passing a current through the solution using inert electrodes. A gas evolves at each electrode, and there is a large increase in pH of the solution. Write the half-reactions that occur at the anode and at the cathode.arrow_forwardConsider a galvanic cell for which the anode reaction is 3 Pb(s)Pb2+(1.0102M)+2e and the cathode reaction is VO2+(0.10M)+2H3O+(0.10M)+eV3+(1.0105M)+3H2O(l) The measured cell potential is 0.640 V. Calculate E for the VO2+V3+ half-reaction, usingE(Pb2+Pb) from Appendix E. Calculate the equilibrium constant (K) at 25°C for thereaction Pb(s)+2VO2+(aq)+4H3O+(aq)Pb2+(aq)+2V3+(aq)+6H2O(l)arrow_forwardSketch the galvanic cells based on the following half-reactions. Show the direction of electron flow, show the direction of ion migration through the salt bridge, and identify the cathode and anode. Give the overall balanced equation, and determine for the galvanic cells. Assume that all concentrations are 1.0 M and that all partial pressures are 1.0 atm. a. b.arrow_forward
- a Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forwardConsider a voltaic cell in which the following reaction takes place in basic medium at 25°C. 2NO3-(aq)+3S2(aq)+4H2O3S(s)+2NO(g)+8OH(aq) (a) Calculate E°. (b) Write the Nernst equation for the cell E. (c) Calculate E under the following conditions: PNO=0.994atm,ph=13.7,[S2]=0.154M,[NO3-]=0.472M, .arrow_forwardUse the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the reaction proceeds spontaneously as written or spontaneously in the reverse direction under standard-state conditions. (a) H2(g)+Cl2(g)2H+(aq)+2Cl(aq) (b) Al3+(aq)+3Cr2+(aq)Al(s)+3Cr3+(aq) (c) Fe2+(aq)+Ag+(aq)Fe3+(aq)+Ag(s)arrow_forward
- A half-cell that consists of a copper wire in a 1.00 M Cu(NO3)2 solution is connected by a salt bridge to a solution that is 1.00 M in both Pu3+ and Pu4+, and contains an inert metal electrode. The voltage of the cell is 0.642 V, with the copper as the negative electrode. (a) Write the half-reactions and the overall equation for the spontaneous chemical reaction. (b) Use the standard potential of the copper half-reaction, with the voltage of the cell, to calculate the standard reduction potential for the plutonium half-reaction.arrow_forwardA galvanic cell is constructed in which the overall reactionis Cr2O72(aq)+14H2O+(aq)+6I(aq)2Cr3+(aq)+3I2(s)+21H2O(l) Calculate E for this cell. At pH 0, with [Cr2O72]=1.5M and [I]=0.40M, the cell potential is found to equal 0.87 V. Calculatethe concentration of Cr3+(aq) in the cell.arrow_forwardThe mass of three different metal electrodes, each from a different galvanic cell, were determined before and after the current generated by the oxidation-reduction reaction in each cell was allowed to flow for a few minutes. The first metal electrode, given the label A, was found to have increased in mass; the second metal electrode, given the label B, did not change in mass; and the third metal electrode, given the label C, was found to have lost mass. Make an educated guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





