
Concept explainers
a)
Interpretation:Silver metal is product of cell given below then whether given cell is galvanic or electrolytic cell should be determined. Also, cathode, anode, and direction of flow of electrons should be determined.
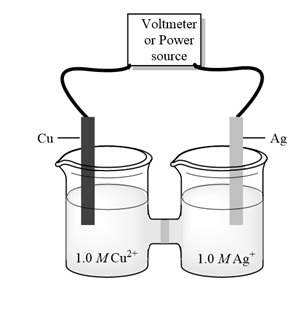
Concept introduction:
b)
Interpretation:Copper metal is product of cell given below then whether given cell is galvanic or electrolytic cell should be determined. Also, cathode, anode, and direction of flow of electrons should be determined.

Concept introduction:Electrolysis is method to transform electrical energy into chemical energy. Electrolytic cells transform electric energy into a chemical energy. In these cells, non-spontaneous reactions occur so energy is supplied for their occurrence. These cells have positive charged anode and negative cathode.
c)
Interpretation:Standard potential of galvanic cell below should be determined.
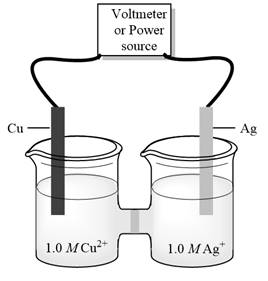
Concept introduction:Electrolysis is method to transform electrical energy into chemical energy. Electrolytic cells transform electric energy into a chemical energy. In these cells, non-spontaneous reactions occur so energy is supplied for their occurrence. These cells have positive charged anode and negative cathode.
d)
Interpretation:Minimum external potential needed to cause reaction to occursin electrolytic cell given below should be determined.
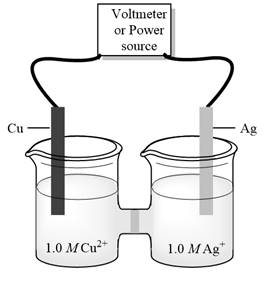
Concept introduction:Electrolysis is method to transform electrical energy into chemical energy. Electrolytic cells transform electric energy into a chemical energy. In these cells, non-spontaneous reactions occur so energy is supplied for their occurrence. These cells have positive charged anode and negative cathode.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Chemical Principles
- find K, the equilibrium constant, if the inital concentration of SO3 is 0.166 M, and the equilibrium concentration of O2 is 0.075 M. 2SO3 (g) ⇌ 2SO2 (g) + O2 (g)arrow_forwardQ4: Rank the relative nucleophilicity of halide ions in water solution and DMF solution, respectively. F CI Br | Q5: Determine which of the substrates will and will not react with NaSCH3 in an SN2 reaction to have a reasonable yield of product. NH2 Br Br Br OH Brarrow_forwardQ7: Rank the following groups in order of basicity, nucleophilicity, and leaving group ability. a) H₂O, OH, CH3COOT b) NH3, H₂O, H₂Sarrow_forward
- Q8: Rank the following compounds in order of increasing reactivity in a nucleophilic substitution reaction with CN as the nucleophile. Br A B NH2 LL F C D OH CI LLI E Q9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d). a) H "Cl D + -OCH 3 Page 3 of 5arrow_forwardQ10: (a) Propose a synthesis of C from A. (b) Propose a synthesis of C from B. Br Br ...\SCH 3 A B Carrow_forward9: Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forward
- Complete the missing entities for following reactions (e.g., major product(s), reactants, and/or solvents) for the SN2 reactions to occur efficiently. Include curved-arrow mechanism for reactions a) to d).arrow_forwardQUESTION 3: Provide the synthetic steps that convert the starting material into the product (no mechanism required). HO OH NH CH3 multiple steps 요요 H3Carrow_forwardQ6: Predict the effect of the changes given on the rate of the reaction below. CH3OH CH3Cl + NaOCH3 → CH3OCH3 + NaCl a) Change the substrate from CH3CI to CH31: b) Change the nucleophile from NaOCH 3 to NaSCH3: c) Change the substrate from CH3CI to (CH3)2CHCI: d) Change the solvent from CH3OH to DMSO.arrow_forward
- Q3: Arrange each group of compounds from fastest SN2 reaction rate to slowest SN2 reaction rate. a) CI Cl فيكم H3C-Cl A B C D Br Br b) A B C Br H3C-Br Darrow_forwardQ2: Group these solvents into either protic solvents or aprotic solvents. Acetonitrile (CH3CN), H₂O, Acetic acid (CH3COOH), Acetone (CH3COCH3), CH3CH2OH, DMSO (CH3SOCH3), DMF (HCON(CH3)2), CH3OHarrow_forwardSuppose the rate of evaporation in a hot, dry region is 1.76 meters per year, and the seawater there has a salinity of 35 ‰. Assuming a 93% yield, how much salt (NaCl) can be harvested each year from 1 km2 of solar evaporation ponds that use this seawater as a source?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





