
(a)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Concept introduction:
Formation of
The
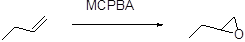
In the nucleophilic substitution reaction, the
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(b)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
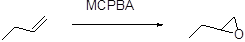
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(c)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
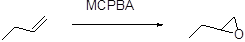
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
(d)
Interpretation:
The stereo isomeric product should be given, when it is reacts with per oxyacid followed by hydroxide ion.
Formation of epoxide:
The alkene can be converted to epoxide, when it is treated with MCPBA (m-chloro perbenzoic acid)
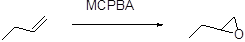
In the nucleophilic substitution reaction, the rate of reaction depends on reactant as well as nucleophile, which are involved in reaction is called bimolecular nucleophilic substitution reaction.
In
Reactant and nucleophile are present at the rate determination step.
The order of species involving in
Tertiary < Secondary < Primary
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Organic Chemistry (8th Edition)
- Pleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardThe Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





