
(a)
Interpretation:
The 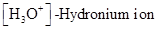 and
and 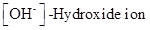 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
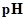 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 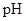 scale. The
scale. The 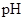 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
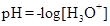
If the value of 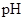 is less than
is less than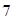 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 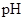 is greater than
is greater than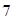 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 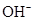 concentration present in the solution.
concentration present in the solution.
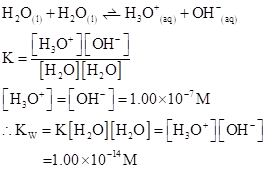
The 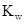 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 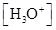 is large that is
is large that is 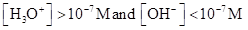
For basic solution 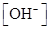 is large that is
is large that is 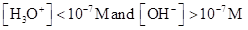
(b)
Interpretation:
The 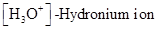 and
and 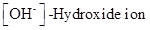 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
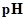 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 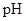 scale. The
scale. The 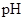 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
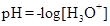
If the value of 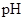 is less than
is less than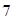 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 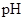 is greater than
is greater than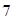 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 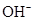 concentration present in the solution.
concentration present in the solution.
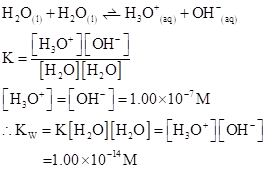
The 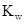 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 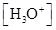 is large that is
is large that is 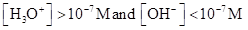
For basic solution 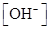 is large that is
is large that is 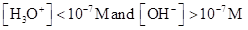
(c)
Interpretation:
The 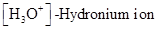 and
and 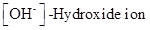 values of given solution should be determined and the most acidic and basic solution should be identified.
values of given solution should be determined and the most acidic and basic solution should be identified.
Concept Introduction:
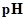 : The concentration of hydrogen ion is measured using
: The concentration of hydrogen ion is measured using 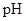 scale. The
scale. The 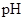 of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
of a solution is a figure that expresses the acidity or the alkalinity of a given solution.
It is defined as the negative base-10 logarithm of the hydrogen or hydronium ion concentration.
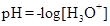
If the value of 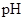 is less than
is less than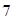 , then the solution is acidic whereas if the value of
, then the solution is acidic whereas if the value of 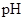 is greater than
is greater than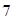 , then the solution is basic.
, then the solution is basic.
Ionic-product constant for water: It is the hydronium ion concentration times the 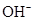 concentration present in the solution.
concentration present in the solution.
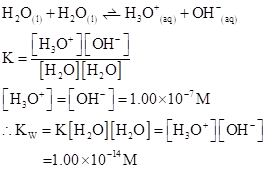
The 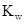 will apply to all aqueous solution.
will apply to all aqueous solution.
For acidic solution 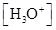 is large that is
is large that is 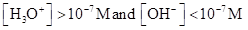
For basic solution 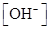 is large that is
is large that is 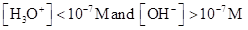
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- You need to use a dilute hydrochloric acid solution in an experiment. However, the only bottle of hydrochloric acid in your lab's acid-base cabinet is 11 M. Calculate the pH of the solution you prepare by diluting 1.5 mL of the 11 M HCl to a final volume of 500 mL with H₂O. pH =arrow_forwardThree buffers are made by combining a 1M solution of acetic acid and a 1M solution of sodium acetate in the ratios shown in the table below. Which of these statements is true regarding the prepared buffers? (Ka= 1.7x10-5) pH of buffer 1 < pH of buffer 2 < pH of buffer 3 pH of buffer 1 = pH of buffer 2 = pH of buffer 3 pH of buffer 1 > pH of buffer 2 > pH of buffer 3 pH of buffer 1 = pH of buffer 2 > pH of buffer 3 pH of buffer 1 > pH of buffer 2 = pH of buffer 3arrow_forwardA buffer solution is composed of 1.00 mol of acid and 1.75 mol of the conjugate base. If the pKa of the acid is 3.60, what is the pH of the buffer? pH=arrow_forward
- What is the pH of a 0.25 M solution of acetic acid, Ka = 1.8 x 105. What percentage of the acid is dissociated?arrow_forwardThe following are pH values for common household substances: swimming pool tile cleaner 2 vinegar 3 coffee 5 blood 7.4 liquid drain cleaner 10 Comparing the pH of tile cleaner and coffee: O a) The [H+] of tile cleaner is 10,000 times higher. b) The [H+] of coffee is three times higher. c) The [H+] of coffee is 1,000 times higher. O d) The [H+] of tile cleaner is 1,000 times higher. O e) The [H+] of tile cleaner is three times higher.arrow_forwardHow much water must be added to 300 mL of an aqueous solution of 0.2 M acetic acid in order to double the degree of ionization? Take the acid ionization constant of acetic acid to be 1.8 x 10-5.arrow_forward
- A solution has a hydrogen ion concentration of 0.01 mol/L. What is its pH? What is its hydroxide ion concentration? Is it acidic, basic, or neutral? How does the hydrogen ion concentration of this solution differ from one with a pH of 1?arrow_forwardYou need to prepare an acetate buffer of pH 5.43 from a 0.621 M acetic acid solution and a 2.95 M KOH solution. If you have 730 mL of the acetic acid solution, how many milliliters of the KOH solution do you need to add to make a buffer of pH 5.43? The pKa of acetic acid is 4.76. Be sure to use appropriate significant figures.arrow_forwardCalculate the pH of a buffer that contains 0.75 M acetic acid and 0.35 M acetate ion in 1 L solution. What will the pH of the buffer be upon the addition of 100.0 mL of 1.0 M HCI? (pKa of acetic acid 4.76)arrow_forward
- If 0.752 moles of (NH3OH)Cl is dissolved in 1 L of water what is the pH of the solution?arrow_forwardWhat is the pH of a solution of 100 ml of 0.01 M H3PO4 and 100 ml of 0.01 M Na3PO4?arrow_forwardIf the pH of a voledronic acid solution is 5.8, and the voledronate concentration is 9 mM, what is the concentration of voledronic acid? (pKa=5.0) 1.4 0.5 185.2 20 379.5arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





