
Prescott's Microbiology
11th Edition
ISBN: 9781260211887
Author: WILLEY, Sandman, Wood
Publisher: McGraw Hill
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.1, Problem 1MI
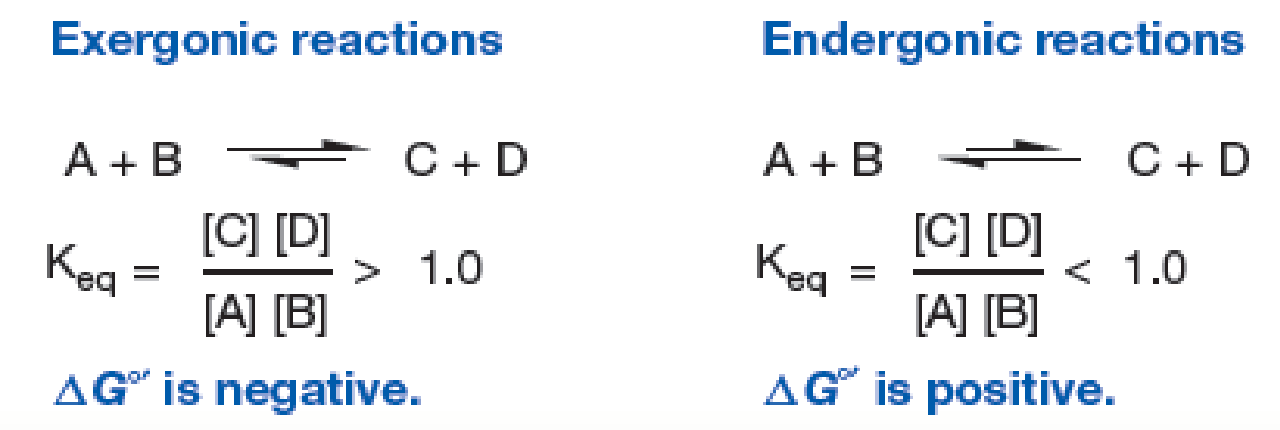
Figure 10.2 The Relationship of ΔG°′ to the Equilibrium of Reactions. Note the differences between exergonic and endergonic reactions.
Which reaction would release heat? Explain your answer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A particular reaction has a ΔG‡ of 37.0 kJ mol-1. In the presence of an enzyme, the same reaction has a ΔG‡ of 5.70 kJ mol-1. Calculate the value of ΔΔG‡ in kJ mol-1.
Figure 1
Figure 2
Reaction
Reaction
Abe notices that the temperature of the mixture in figure 1 gets
colder.
Abe nota que la temperatura de la mezcla en la figura 1 se vuelve más
fría.
A) Describe the systems (i.e. in the reaction and its surroundings), in
which the energy is conserved, represented by the two figures.
B) Explain why energy is neither created nor destroyed in these
systems.
Energy-
Energy
Which one of the following statements is completely TRUE?
O When AG > 0, the reaction is BOTH product-favored (spontaneous) AND endergonic.
When AG 0, the reaction is BOTH reactant-favored (nonspontaneous) AND endergonic.
When AG > 0, the reaction is BOTH product-favored (spontaneous) AND exergonic.
When AG > 0, the reaction is BOTH reactant-favored (nonspontaneous) AND exergonic.
When AG < 0, the reaction is BOTH reactant-favored (nonspontaneous) AND exergonic.
Chapter 10 Solutions
Prescott's Microbiology
Ch. 10.1 - Figure 10.2 The Relationship of G to the...Ch. 10.1 - Prob. 1CCCh. 10.1 - Prob. 2CCCh. 10.1 - Prob. 3CCCh. 10.1 - Prob. 4CCCh. 10.2 - Why is ATP called a high-energy molecule? How is...Ch. 10.2 - Describe the energy cycle and ATPs role in it....Ch. 10.3 - Prob. 1MICh. 10.3 - Prob. 2MICh. 10.4 - Figure 10.6 Electron Movement and Reduction...
Ch. 10.4 - How is the direction of electron flow between...Ch. 10.4 - When electrons flow from the NAD+/NADH conjugate...Ch. 10.4 - Which among the following would be the best...Ch. 10.4 - In general terms, how is G related to E0? What is...Ch. 10.4 - Name and briefly describe the major electron...Ch. 10.6 - Will an enzyme with a relatively high Km have a...Ch. 10.6 - Prob. 2MICh. 10.6 - Prob. 1CCCh. 10.6 - Prob. 2CCCh. 10.6 - How does enzyme activity change with substrate...Ch. 10.6 - What special properties might an enzyme isolated...Ch. 10.6 - What are competitive and noncompetitive...Ch. 10.6 - How are enzymes and ribozymes similar? How do they...Ch. 10.7 - Figure 10.19 Allosteric Regulation. The structure...Ch. 10.7 - Prob. 2MICh. 10.7 - Define the terms metabolic channeling and...Ch. 10.7 - Define allosteric enzyme and allosteric effector.Ch. 10.7 - Prob. 3CCCh. 10.7 - Prob. 4CCCh. 10.7 - Prob. 5CCCh. 10 - Prob. 1RCCh. 10 - Prob. 2RCCh. 10 - Prob. 3RCCh. 10 - Examine the structures of macromolecules in...Ch. 10 - Examine the branched pathway shown here for the...Ch. 10 - Prob. 3AL
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (10th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- On the free energy diagram shown, label the intermediate (s) and transition state(s). Is the reaction thermodynamically favorable? Reaction.arrow_forwardWhat is an example where molecules or aroma within a cell physically overcome some barrier to their reaction going forward thag is exergonic one? (hint: think about what a catalyst physically does during a reaction).arrow_forwardT/F A catalyst changes only the rate at which equilibrium is achieved; it has no effect on the position of the equilibrium. This means that a catalyst can enhance the rate of exergonic reactions but cannot somehow change the ΔG′ to allow an endergonic reaction to become spontaneousarrow_forward
- this one represents an endothermic reaction. Things are similar: the flat line on the left (beginning of the reaction) is the total energy possessed by the reactant molecules; once again, kJ stands for energy in kiloJoules, thousands of Joules. The flat line on the right (reaction complete) is the total energy of the products. Since an endothermic reaction has a net absorption of energy (taking this extra energy from the surroundings), the products have higher energy than the reactants. Question: the energy of the reactant molecules is kJ. [to answer, simply identify the correct y-axis coordinate.] 250 200 PE (kJ) 150 100 50 Reaction pathwayarrow_forwardDetermine the direction that each of the reactions will progress. Assume that the reactants and products are present in equimolar amounts. The standard free energy of hydrolysis of ATP is - 30.5 kJ/mol. fructose + ATP fructose 6-phosphate + ADP The standard free energy of hydrolysis for fructose 6-phosphate is -15.9 kJ/mol. 3-phosphoglycerate + ATP 1,3-bisphosphoglycerate + ADP The standard free energy of hydrolysis for 1,3-bisphosphoglycerate is -49.3 kJ/mol. creatine + ATP creatine phosphate + ADP The standard free energy of hydrolysis for creatine phosphate is -43.0 kJ/mol.arrow_forwardif the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established A + B ⇌ C a. the concentration of C would increase for a time, then remain constant b. the concentration of A would increase for a time, then decrease c. the concentration of B would increase for a time, then remain constantarrow_forward
- Consider the reaction below to answer the following question(s): + HBr A B Br с + D Br Enter the appropriate letter in the blank for each the following statements. The kinetically controlled product in this reaction is D B Aarrow_forwardThe following questions are based on the reaction A+ B ↔ C+D shown in Figure 8.1. 1. Which of the following terms best describes the progress of the reaction with respect to free energy change? a) endergonic, ∆G> 0 b) exergonic, ∆G> 0 c) exergonic, ∆G< 0 d) endergonic, ∆G< 0 2. Which of the following in Figure 8.1 remains unchanged by having an enzyme included? a) b b) d c) a d) c 3. The part labeled “C” on the above graph represents a) Energy of activation without enzyme b) Energy of activation with enzyme c) Amount of free energy released d) amount of energy required for the reaction progressarrow_forwardThe primary source of phosphate for chemical reactions in cells comes as ATP, ADP, or AMP. The ΔG˚’ for the hydrolysis of ATP into ADP and Pi is -30.5 kJ/mol. Calculate the equilibrium constant for the hydrolysis of ATP.arrow_forward
- Consider the following endothermic reaction at equilibrium: H2(g) + Zn2+(aq) ⇌ Zn(s) + 2 H+(aq) Which of the following disturbances will result in the reaction going forward to re-establish equilibrium? (Select all that apply.) None of these Correct Answer Increase temperature at constant pressure. Correct! Add liquid water. Decrease in partial pressure of H2(g). Decrease container volume at constant temperature. Adding the strong acid, HNO3. (Consider any change in solution volume is negligible.) Removing some solid zinc. Adding the soluble salt, magnesium nitrate, Zn(NO3)2. Adding the soluble salt, sodium sulfide, Na2S.arrow_forwardShow how the Michaelis-Menten Equation was derived from this reaction. k1 k2 E + S = e ES E + Parrow_forwardThe following exothermic reaction is at 0.00 °C and 1.00 atm SeO4 (g) ⇌ Se(g) + O2(g) , kc = 2.4 ×10-6 The reaction contains [SeO4] = 0.100 M, [Se] = 0.0034 M, [O2] = 0.0022 M Does the reaction exist at equilibrium? If not, in what direction it will proceed? Question 22 options:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax
The Cell Membrane; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=AsffT7XIXbA;License: Standard youtube license