
Concept explainers
Convert each molecule into a skeletal structure.
a.
b.
c.
d.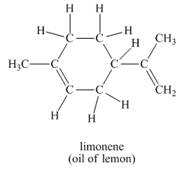
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Organic Chemistry
Fundamentals Of Thermodynamics
General, Organic, and Biological Chemistry - 4th edition
Human Anatomy & Physiology (2nd Edition)
- What is a hydrocarbon? What is the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? Distinguish between normal and branched hydrocarbons. What is an alkane? What is a cyclic alkane? What are the two general formulas for alkanes? What is the hybridization of carbon atoms in alkanes? What are the bond angles in alkanes? Why are cyclopropane and cyclobutane so reactive? The normal (unbranched) hydrocarbons are often referred to as straight-chain hydrocarbons. What does this name refer to? Does it mean that the carbon atoms in a straight-chain hydrocarbon really have a linear arrangement? Explain. In the shorthand notation for cyclic alkanes, the hydrogens are usually omitted. How do you determine the number of hydrogens bonded to each carbon in a ring structure?arrow_forwardClassify the following hydrocarbons as alkanes, cycloalkane, alkene, cycloalkenes, alkyne, cycloalkyne, or aromatic.a. CH2 CH CH2b. CH3 C (CH3)2 CH (CH2CH3) CH2 CH2 CH (CH3)2c. (CH3)3 C C C CH (CH3) CH2 CH3arrow_forward1. Identify the circled functional group(s) for each molecule. ОН CH3CHCH3 a. бо =CH₂ b. с. d. NH₂CH₂CH₂CH₂2NHCH3 H₂N 0 CH₂CHCH₂NHCH(CH3)2 стилинен с OHarrow_forward
- Please answer both questionsarrow_forwardFor each molecule, draw the structure and indicate whether cis/trans isomers are possible. If they do, draw them both and label them as cis and trans. a. 2-methyl-1-butene b.1,2-dimethylcyclohexanearrow_forwardWhich statement of the following is false? A. Cyclohexene has the general formula CH20-2 B. Alkenes are also referred to as olefins O C. The alkene C atoms are sp2 hybridized and have a bond angle of 120°C D. Trans isomers are identical to (Z) isomers E. The bond angle around the alkyne C atoms is 180°Carrow_forward
- 2. Draw the mechanism of the bromination of acetylene: 3. Draw the structures of the following: a. 3-bromo-3,4-dimethyl-1-hepten-5-yne b. Acetylene c. 1-ethynyl-2-methylcyclohexanearrow_forwardDraw skeletal structures for the compounds including any cis–trans isomers.arrow_forward1.Draw the structural formula of CH 3 CH(CH 3 )(CH 2 ) 4 CH3 2. How does the general formula of a cycloalkane compare to that of an alkane?arrow_forward
- What is the purpose of the crystallization technique in organic chemistry? to run a chemical reaction that will yield a unique product to determine whether a compound is soluble in a specific solvent a purification technique for impure solids a way to turn a liquid compound into a solidarrow_forwarda. How many Lewis structures have the formula C4H11N? 10 b. In how many of the structures is the nitrogen atom attached to only one carbon? c. How many of them have carbon-carbon double bonds? d. How many of them have carbon-nitrogen double bonds? e. How many of them have rings? f. How many of the structures are capable of hydrogen bonding? g. How many of the structures contain a carbon atom attached to three other carbons? h. How many of the structures contain a nitrogen atom attached to three carbons? Varrow_forward9. Classify alcohols as primary (1°), secondary (2°), or tertiary (3°). Functional Organic family 1°, 2°, 3° Formula Compound name group name CH3-CH2-CH-CH2-OH Alcohol group CH3 CH,-CH,-0-CH,-CH,-CH, CH,-CH2-C-H CH2-CH, CH3-C-CH2-CH2-CH3 OH CH- CH- CH- CH- COOH CH3 HO.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning  Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




