
Concept explainers
a)
Interpretation: Among below experiments I, II, III and IV; experiments that yield same average result should be determined.
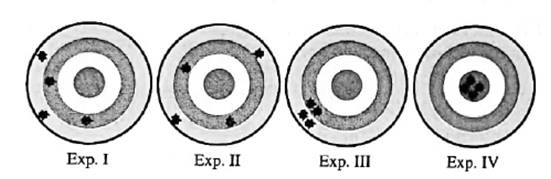
Concept introduction:Any test, trial or tentative procedure that is executed under highly controlled conditions whose purpose is to examine validity of any hypothesis is called an experiment. These are also performed so as to discover unknown things in nature.
Below mentioned are some of the essential features of a well-designed experiment.
1. Experiment should be designed in such a way that it can be repeated to have same results.
2. Such variables should be used in experiments that can be easily controlled and manipulated directly or indirectly by the performer.
3. Atleast two relatable variables should be present in experiment.
b)
Interpretation: Among below experiments I, II, III and IV; experiments that displayhigh precision should be determined.
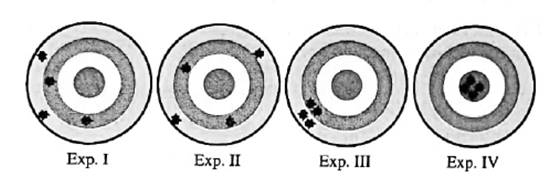
Concept introduction:Any test, trial or tentative procedure that is executed under highly controlled conditions whose purpose is to examine validity of any hypothesis is called an experiment. These are also performed so as to discover unknown things in nature.
The closeness of measurements with each other is termed as precision. It determines the extent to which measurements are close to each other.
c)
Interpretation: Among below experiments I, II, III and IV; experiments that display high accuracy should be determined.
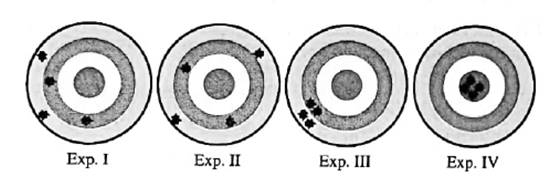
Concept introduction:Any test, trial or tentative procedure that is executed under highly controlled conditions whose purpose is to examine validity of any hypothesis is called an experiment. These are also performed so as to discover unknown things in nature.
The extent to which calculated or measured values are close to standard one is called accuracy.
d)
Interpretation: Among below experiments I, II, III and IV; experiments that show systematic error should be determined.
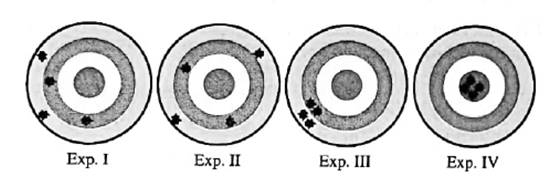
Concept introduction:Any test, trial or tentative procedure that is executed under highly controlled conditions whose purpose is to examine validity of any hypothesis is called an experiment. These are also performed so as to discover unknown things in nature.
Errors in any measurement can be either random or systematic. These are mentioned below.
1. Systematic errors: Such errors arise due to faulty experimental set or equipment.
2. Random errors: These occur due to precision of the instruments.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Principles of General Chemistry
- The label on a bale of mulch indicates a volume of 1.45 ft3. The label also states that the mulch in the bale will cover an area of a garden 6 ft 6 ft to a depth of 1 in. Account for the discrepancy in the given volumes.arrow_forwardWhich of the following represent physical properties or changes, and which represent chemical properties or changes? You curl your hair with a curling iron. You curl your hair by getting a “permanent wave” at the hair salon. Ice on your sidewalk melts when you put salt on it. A glass of water evaporates overnight when it is left on the bedside table. Your steak chars if the skillet is too hot. Alcohol feels cool when it is spilled on the skin. Alcohol ignites when a flame is brought near it. Baking powder causes biscuits to rise.arrow_forwardIn the following scenario, identify which of the statements represents a theory, law, or hypothesis. (a) A student exploring the properties of gases proposes that is she decreases the volume of a sample of gas then the pressure exerted by the sample will increase (b) Many scientists over time have conducted similar experiments and have concluded that pressure and volume are inversely proportional. (c) She proposes that the reason this occurs is that if the volume is decreased, more molecules will collide with a given area of the container walls, causing the pressure to be greater.arrow_forward
- Identify each of the underlined items as a part of either the macroscopic domain, the microscopic domain, or the symbolic domain of chemistry. For any in the symbolic domain, indicate whether they are symbols for a macroscopic or a microscopic feature.(a) The mass of a lead pipe is 14 lb.(b) The mass of a certain chlorine atom is 35 amu.(c) A bottle with a label that reads Al contains aluminum metal.(d) Al is the symbol for an aluminum atom.arrow_forwardThe density of a certain metal is (6.60x10^0) g/cm3. What is the mass in grams of a sample of this metal that has a volume of (8.460x10^-2) m3? Enter your answer in scientific notation with 3 sig figs. Do not include any units in your answer. Do not round any intermediate calculations. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer:arrow_forwardThe following statements pertain to the development of the theory of combustion by the French chemist Lavoisier in the eighteenth century. Match the statement with the appro- priate step (observation, hypothesis, experiment designed to test hypothesis) in the scientific method. (a) A metal is burned in a closed container, and the change in mass of the solid and volume of the gas is measured.(b) Oxygen gas combines with a substance during its combustion.(c) Combustion of a metal in a closed container ceases after a length of time.arrow_forward
- If a sample of gold alloy has a mass of 87.7 g and is 78.3% gold by mass, what percentage of the alloy is made up of other metals? Write your answer to the nearest tenth of a percent. (I had to alter the wording of this question since it wasn't calculating significant figures properly, so if you got it wrong because of significant figures you can assume you probably actually got it correct.)arrow_forwardThe accepted value of the melting point of pure aspirin is 135 °C. Trying to verify that value, you obtain 134 °C, 136 °C, 133 °C, and 138 °C in four separate trials. Your partner finds 138 °C, 137 °C, 138 °C, and 138 °C. (a) Calculate the average value and percent error for your data and your partner’s data. (b) Which of you is more precise? More accurate?arrow_forwardImagine that have a cube of iron metal that you know has a mass of exactly 1.0000 grams because the cube was calibrated before you purchased it. You carefully measure the mass of this cube several times using the balance in your lab, but every time the balance indicates that the cube has a mass of 1.0900 grams. (i) State whether this balance be considered to be accurate, precise, both, or neither and (ii) explain how you made your choice.arrow_forward
- Take the mass measurement with nitrogen (28 dalton or atomic mass units per molecule) and the mass measurement with helium (4 daltons per atom) and use the difference between the measured masses to find the mass of 1 dalton (one atomic mass unit) in grams. The standard value of the dalton is 1.66053906660×10−27 kg with an uncertainty of ±50 in the last two figures. Can you identify the sources of error in your measurement?arrow_forwardTwo beakers contain clear, colorless liquids. When the contents of the beakers are mixed, a white solid is formed. (a) Is this an example of a chemical or a physical change? (b) What would be the most convenient way to separate the newly formed white solid from the liquid mixture— filtration, distillation, or chromatography. Elaborate your answer.arrow_forwardA student gently drops an object weighing 16.5 g into an open vessel that is full of ethanol, so that a volume of ethanol spills out equal to the volume of the object. The experimenter now finds that the vessel and its contents weigh 10.5 g more than the vessel full of ethanol only. The density of ethanol is 0.789 g / cm^3. What is the density of the object?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



