
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
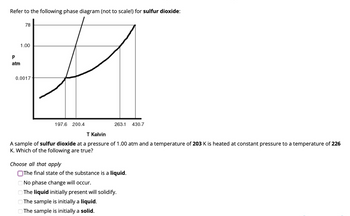
Transcribed Image Text:Refer to the following phase diagram (not to scale!) for sulfur dioxide:
78
1.00
P
atm
0.0017
197.6 200.4
263.1
Choose all that apply
T Kelvin
A sample of sulfur dioxide at a pressure of 1.00 atm and a temperature of 203 K is heated at constant pressure to a temperature of 226
K. Which of the following are true?
430.7
The final state of the substance is a liquid.
No phase change will occur.
The liquid initially present will solidify.
The sample is initially a liquid.
The sample is initially a solid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Study the following phase diagram of Substance X. pressure (atm) 36- 18- solid 0 100 liquid 200 gas temperature (K) Use this diagram to answer the following questions. Sunnose a small sample of pure X is held at -104 C and 19.6 atmarrow_forwardUse the phase diagram of Substance X below to find the pressure at which the melting point of X is - 173. °C. 1.6- solid liquid 0.8- gas 100 200 temperature (K) Note: your answer must be within 0.1 atm of the exact answer to be graded correct. atm pressure (atm)arrow_forwardThe pressure above a pure sample of solid Substance X at -10. °C is lowered. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 04- 02- 6 0 atm solid liquid 400 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. gas 600arrow_forward
- Nonearrow_forwardThe pressure above a pure sample of solid Substance X at -5. °C is lowered. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. 0.8- solid ol liquid 0.4 18 Ar gas 0. 200 400 600 temperature (K) Note: your answer must be within 0.05 atm of the exact answer to be graded correct. D atm pressure (atm)arrow_forward[References) Use the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for xenon: 57.6 1.00 atm 0.37 152.0 161.3 165.0 289.7 T Kelvin A sample of xenon at a pressure of 0.370 atm and a temperature of 140 K is compressed at constant temperature to a pressure of 66.5 atm. Which of the following are true? Choose all that apply | The sample is initially a gas. O No phase change will occur. O The solid initially present will vaporize. O The final state of the substance is a solid. Previous O The sample is initially a solid. En rcharrow_forward
- Refer to the following phase diagram (not to scale!) for sulfur dioxide: P atm 78 1.00 0.0017 197.6 200.4 Choose all that apply T Kelvin A sample of sulfur dioxide at a pressure of 1.70×10-3 atm and a temperature of 266 K is compressed at constant temperature to a pressure of 83.7 atm. Which of the following are true? 263.1 430.7 The sample is initially a gas. The liquid initially present will vaporize. The final state of the substance is a liquid. The sample is initially a solid. One or more phase changes will occur.arrow_forwardUse the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for oxygen: 49.8 1.00 P atm 0.00150- 54.4 54.8 90.2 154.6 T Kelvin A sample of oxygen at a pressure of 1.00 atm and a temperature of 48.6 K is heated at constant pressure to a temperature of 99.9 K. Which of the following are true? Choose all that apply OThe gas initially present will solidify. O One or more phase changes will occur. The sample is initially a solid. O The sample is initially a liquid. O The final state of the substance is a gas. Previous Nextarrow_forwardThe enthalpy of vaporization of Substance x is 7.00 kJ/mol and its normal boiling point is -10c . Calculate the vapor pressure of x at -36c. Round your answer to 2 significant digitsarrow_forward
- At constant pressure p1, the substance goes from solid state to gas as temperature increases At constant temperature T1, the substance goes from gas state to liquid as pressure increases The substance exists in the liquid phase by lowering the pressure and the temperature beyond T At temperature T, the substance coexists in solid, liquid and gas phases At constant pressure p2, the substance goes from solid state to liquid to gas as temperature increases.arrow_forwardA sample of neon at a pressure of 1.00 atm and a temperature of 18.3 K is heated at constant pressure to a temperature of 79.5 K. Which of the following are true?Choose all that apply The sample is initially a solid. One or more phase changes will occur. The gas initially present will solidify. The sample is initially a liquid. The final state of the substance is a gas.arrow_forward$ 4 R F [Review Topics] Refer to the following phase diagram (not to scale!) for neon: P atm 26.2 1 1.00 V 0.425 Choose all that apply T Kelvin A sample of neon at a pressure of 1.00 atm and a temperature of 26.3 K is heated at constant pressure to a temperature of 51.6 K. Which of the following are true? 5 The liquid initially present will solidify. The final state of the substance is a gas. The sample is initially a solid. One or more phase changes will occur. The sample is initially a liquid. T G 244 24.5 E B Cengage Learning Cengage Technical Support 6 MacBook Air Y H 27.1 44.4 & 7 N U J 8 M D-II 1 K ( 9 H 4 [References] O ) O L P 18 command A . ; option { [ Previous + ? 11 ✰ ✰ O Next> Save and Exit Ne X 1 delete 1: S + Update V D (?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY