
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
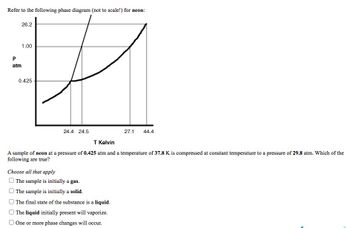
Transcribed Image Text:Refer to the following phase diagram (not to scale!) for neon:
26.2
P
atm
1.00
0.425
24.4 24.5
27.1
44.4
T Kelvin
A sample of neon at a pressure of 0.425 atm and a temperature of 37.8 K is compressed at constant temperature to a pressure of 29.8 atm. Which of the
following are true?
Choose all that apply
The sample is initially a gas.
The sample is initially a solid.
The final state of the substance is a liquid.
The liquid initially present will vaporize.
One or more phase changes will occur.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Molecular Solid: A solid material primarily held in this phase by intermolecular forces causing attraction between the molecules. True Falsearrow_forwardLL The vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure -27. °C 0.0449 atm -16. °C 0.112 atm -5.°C 0.257 atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. Continue O 2022 McGraw Hill LLC. All Rights Re EPIC MacBook Pro ロ号 F3 F4 F5 F2 24 4. 23 7. 6. 3. 5. A G D. B. N 8arrow_forward#9 Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point? A. water, bp = 100°C B. methanol, bp = 65°C C. benzene, bp = 80°C D. ethanol, bp = 78°C E. The vapor pressure of each of the liquids at its normal boiling point would be the same.arrow_forward
- A sample of argon at a pressure of 0.680 atm and a temperature of 76.4 K is compressed at constant temperature to a pressure of 50.6 atm. Which of the following are true?Choose all that apply The sample is initially a gas. The final state of the substance is a solid. No phase change will occur. The solid initially present will vaporize. The sample is initially a solid.arrow_forward[References) Use the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for xenon: 57.6 1.00 atm 0.37 152.0 161.3 165.0 289.7 T Kelvin A sample of xenon at a pressure of 0.370 atm and a temperature of 140 K is compressed at constant temperature to a pressure of 66.5 atm. Which of the following are true? Choose all that apply | The sample is initially a gas. O No phase change will occur. O The solid initially present will vaporize. O The final state of the substance is a solid. Previous O The sample is initially a solid. En rcharrow_forwardA 250 mL flask contains air at 1.005 atm and 24.7 °C.5 mL of ethanol is added, the flask is immediately sealed and then warmed to 95.5 °C, during which time a small amount of the ethanol vaporizes. The final pressure in the flask (stabilized at 95.5 °C) is 2.876 atm. a) What is the partial pressure of air, in the flask at 95.5 °C? b)A solution which contains 77.5 g of an unknown molecular compound in 342 g of water freezes at -5.18°C.What is the molar mass of the unknown?arrow_forward
- The statements in the following multiple choices deal with various aspects of the relationship between vapor pressure and intermolecular forces. Choose the statement which is false. a) Volume has no effect on the vapor pressure of water. b) At a given temperature liquid HCl has higher vapor pressure than liquid HF. c) CHBr3 is less volatile than CHCl3. d) At a given temperature solid SF6 has higher vapor pressure than solid I2. e) The average kinetic energy of boiling water molecules is the same on a mountaintop as it is at sea level.arrow_forwardPlease solve the below questions using the attached diagram: If I had a quantity of this substance at a pressure 2.0 atm and a temperature of -150 degrees celsius, what phas change(s) would occur if I decreased the pressure to 0.25 atm? At what pressure(s) would they occur? (Note: multiple answers needed for this question)arrow_forwardTry Again Your answer is incorrect. The enthalpy of vaporization of Substance X is 23.0 Round your answer to 2 significant digits. 0.32 atm x10 X kJ and its normal boiling point is 10. °C. Calculate the vapor pressure of Xat - 18. °C. mol Sarrow_forward
- Study the following phase diagram of Substance X. pressure (atm) O STATES OF MATTER Using a phase diagram to predict phase at a given temperature... 12 solid Explanation 100 liquid 200 temperature (K) Use this diagram to answer the following questions. Check gas Suppose a small sample of pure X is held at -90. °C and 7.7 atm. What will be the state of the sample? Suppose the temperature is held constant at -90. °C but the pressure is decreased by 5.8 atm. What will happen to the sample? y pearson login - Yahoo Search Results Yahoo Search Results (choose one) (choose one) Aarrow_forwardRefer to the following phase diagram (not to scale!) for sulfur dioxide: P atm 78 1.00 0.0017 197.6 200.4 Choose all that apply T Kelvin A sample of sulfur dioxide at a pressure of 1.70×10-3 atm and a temperature of 266 K is compressed at constant temperature to a pressure of 83.7 atm. Which of the following are true? 263.1 430.7 The sample is initially a gas. The liquid initially present will vaporize. The final state of the substance is a liquid. The sample is initially a solid. One or more phase changes will occur.arrow_forwardUse the References to access important values if needed for this question. Refer to the following phase diagram (not to scale!) for oxygen: 49.8 1.00 P atm 0.00150- 54.4 54.8 90.2 154.6 T Kelvin A sample of oxygen at a pressure of 1.00 atm and a temperature of 48.6 K is heated at constant pressure to a temperature of 99.9 K. Which of the following are true? Choose all that apply OThe gas initially present will solidify. O One or more phase changes will occur. The sample is initially a solid. O The sample is initially a liquid. O The final state of the substance is a gas. Previous Nextarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY