
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
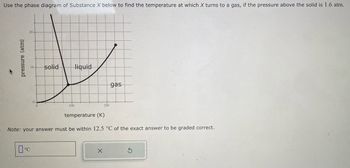
Transcribed Image Text:Use the phase diagram of Substance X below to find the temperature at which X turns to a gas, if the pressure above the solid is 1.6 atm.
pressure (atm)
20-
10- solid
°C
0-
100
liquid
200
temperature (K)
Note: your answer must be within 12.5 °C of the exact answer to be graded correct.
X
gas
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature on a sample of pure X held at 0.53 atm and – 168. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.35 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3 دنا 2- 0. ایل 200 temperature (K) 400 x 5arrow_forwardThe pressure above a pure sample of solid Substance X at -142. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. بالا pressure (atm) 0 0. atm solid liquid 200 temperature (K) Note: your answer must be within 0.125 atm of the exact answer to be graded correct. gas X 400arrow_forwardUse the phase diagram of Substance X below to find the pressure at which the melting point of X is - 173. °C. 1.6- solid liquid 0.8- gas 100 200 temperature (K) Note: your answer must be within 0.1 atm of the exact answer to be graded correct. atm pressure (atm)arrow_forward
- This question as been getting rejected, but this is not a grade assigment. This just a pratice problem.arrow_forwardThe pressure above a pure sample of solid Substance X at -10. °C is lowered. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 04- 02- 6 0 atm solid liquid 400 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. gas 600arrow_forwardNeed answers for the blue parts,thank you!!arrow_forward
- The pressure above a pure sample of solid Substance X at -146. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to find your answer. pressure (atm) solid 200 liquid temperature (K) 400 gas Note: your answer must be within 0.5 atm of the exact answer to be graded correct.arrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X I have been determined: melting point enthalpy of fusion temperature (°C) 120- 110- 100- 90- 80 70- 60- You may also assume X behaves as an ideal gas in the vapor phase. Suppose a small sample of X at 20 °C is put into an evacuated flask and heated at a constant rate until 10.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. density 50+ 40 30- 20 65. °C 10.00 kJ/mol 3 2.80 g/cm³ (solid) 2.30 g/mL (liquid) 0 heat added (kJ/mol) 8 9 boiling point enthalpy of vaporization 10 heat capacity P 100. °C X 21.00 kJ/mol -1 38. J K mol (solid) 29. J.K¹ mol 48. J.K¹ mol 1 (liquid) (vapor) 2 00 ola Ar Barrow_forwardNonearrow_forward
- The vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure 4. °C 0 16. °C 28. °C 0.0584 atm 0.0961 atm 0.152 atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. x10 ロ・ロ X I olo Śarrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure, torr 900- 800- 700- 600- 500- 400. 300. 200- 100- 0 50 60 80 temperature, °C Use the graph to answer the following questions: 70 Which liquid is the most volatile? Which is the least volatile? What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. Suppose a beaker of acetone is put inside a sealed tank containing acetone gas at 54. degree C and 709. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? tetrahydrofuran acetone isopropyl acetate most volatile: least volatile: 90 tetrahydrofuran: acetone: isopropyl acetate: more less the same choose one choose one пос пос [°℃arrow_forwardRefer to the following phase diagram (not to scale!) for hydrogen: 12.8 1.00 atm 0.071 13.9 14.0 20.3 33.2 T Kelvin A sample of hydrogen at a pressure of 7.10x10 atm and a temperature of 11.4 K is compressed at constant temperature to a pressure of 18.2 atm. Which of the following are true? Choose all that apply O The liquid initially present will vaporize. O The final state of the substance is a solid. O The sample is initially a solid. O No phase change will occur. O The sample is initially a gas. Submit Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY