
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
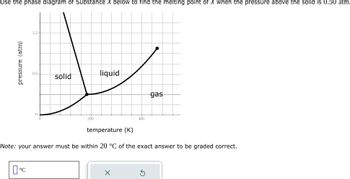
Transcribed Image Text:Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 0.50 atm.
pressure (atm)
1.2-
0.6-
solid
liquid
gas
0.
0
200
400
temperature (K)
Note: your answer must be within 20 °C of the exact answer to be graded correct.
0 °C
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which statments are false? Question is in the imagearrow_forward1) NaOEt 2) Br 3) H3O*, heatarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Ethanol, C2H,O, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The heat of combustion of ethanol is 326.7 kcal/mol. The heat of combustion of hexane, C,H14, is 995.0 kcal/mol. How much energy is released during the complete combustion of 479 grams of hexane ? kcal Assuming the same efficiency, would 479 grams of ethanol provide more, less, or the same amount of energy as 479 grams of hexane? Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- 500.0 mL of 0.100 mol·L HCl(aq) was added to a high quality insulated calorimeter containing 500.0 mL of 0.100 mol·LNaOH(aq). Both solutions had a density of 1.000 g·mL and a specific heat of 4.184 J·g ·K . The calorimeter had a heatcapacity of 850.0 J·K . The temperature of the entire system rose from 25.50 C to 26.05 C.Calculate the heat of reaction. Thank you!arrow_forwardName these organic compounds: Η Η Η Η Η Η ||| C=C=C-H ||| Η Η Η Η Η II H — C =C=C=C=C - H ||||| Η structure Η Η Η Η Η Η H H Η Η Η | Η ||| H – C – C =C=C=C=C=CH ||||||| Η Η Η Η Η Η Η name Π Π Πarrow_forwardarrow_forward
- Give the expression for the Ksp for the following CACO3(s) Ca*2 CO3 2 Ksp = %3Darrow_forwardQ1. Consider the following reaction: 2 KMnO4(aq) + 3 H₂SO4(aq) + 5 H₂O2(aq) Complete the table below (answers must be values with proper signs and units). A[H₂SO4)/At -0.156 mol L ¹5-1 ->> Δ[02]/ΔΙ 0.260 mol L¹ s1 K₂SO4(aq) + 2 MnSO4(aq) + 8 H₂O(1) + 5 02(aq) A[KMnO4]/At Reaction Rate -0.104 mal L'5" 0.052 MOLL'S!arrow_forward-1/3=j/4-10/3 What is j?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY