
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
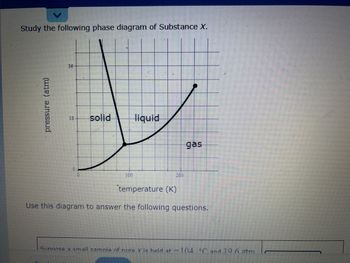
Transcribed Image Text:Study the following phase diagram of Substance X.
pressure (atm)
36-
18- solid
0
100
liquid
200
gas
temperature (K)
Use this diagram to answer the following questions.
Sunnose a small sample of pure X is held at -104 C and 19.6 atm
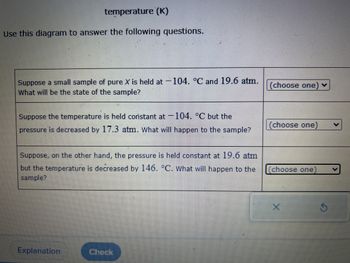
Transcribed Image Text:temperature (K)
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at 104. °C and 19.6 atm.
What will be the state of the sample?
Suppose the temperature is held constant at 104. °C but the
pressure is decreased by 17.3 atm. What will happen to the sample?
Suppose, on the other hand, the pressure is held constant at 19.6 atm
but the temperature is decreased by 146. °C. What will happen to the
sample?
Explanation
Check
(choose one) ✓
(choose one)
choose one
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the observation in the first column to answer the question in the second column. observation At 1 atm pressure, Substance C boils at 5. °C and Substance D boils at -31. °C. The enthalpy of vaporization of Substance A is smaller than that of Substance B. At 23 °C, Substance E has a vapor pressure of 105. torr and Substance F has a vapor pressure of 55. torr. question Which has a higher enthalpy of vaporization? Substance C Substance D Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? Substance E Substance F Neither, E and F have the same boiling point. It's impossible to know without more information.arrow_forwardRefer to the following phase diagram (not to scale!) for oxygen : 49.8 1.00 P atm 0.00150- 54.4 54.8 O₂ is a O₂ is a O₂ is a 90.2 154.6 T Kelvin At a pressure of 1 atm, the temperature 54.8 K is called the The normal boiling point for O₂ is at к The triple point pressure for O₂ is atm The critical pressure for O₂ is 49.8 atm. At temperatures above 154.6 K and pressures above 49.8 atm, O₂ is a ✓at 1.50×10-³ atm and 104 K. ✓at 1.00 atm and 66.8 K. ✓at 45.6 atm and 40.3 K. of 0₂. Prarrow_forwardDifluoroethene has two isomers with very different properties despite having the same chemical formula. Based on the chemical structure shown for the first isomer, what intermolecular forces are present in this molecule?arrow_forward
- This question as been getting rejected, but this is not a grade assigment. This just a pratice problem.arrow_forwardThe pressure above a pure sample of solid Substance X at -10. °C is lowered. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. pressure (atm) 04- 02- 6 0 atm solid liquid 400 temperature (K) Note: your answer must be within 0.025 atm of the exact answer to be graded correct. gas 600arrow_forwardUse the observation in the first column to answer the question in the second column. observation At 1 atm pressure, Substance E boils at 11. °C and Substance F boils at 30. °C. At 37 °C, Substance C has a vapor pressure of 89. torr and Substance D has a vapor pressure of 79. torr. The enthalpy of vaporization of Substance A is smaller than that of Substance B. question Which has a higher vapor pressure? Substance E Substance F Neither, E and F have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance C Substance D Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. X Śarrow_forward
- The boiling point is directly related to the strength of the intermolecular forces between each molecule. Describe the different intermolecular forces that are present in toluene. Which of these intermolecular forces is strongest?arrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: vapor pressure, torr 900- 800- 700- 600- 500- 400. 300. 200- 100- 0 50 60 80 temperature, °C Use the graph to answer the following questions: 70 Which liquid is the most volatile? Which is the least volatile? What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. Suppose a beaker of acetone is put inside a sealed tank containing acetone gas at 54. degree C and 709. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? tetrahydrofuran acetone isopropyl acetate most volatile: least volatile: 90 tetrahydrofuran: acetone: isopropyl acetate: more less the same choose one choose one пос пос [°℃arrow_forwardRefer to the following phase diagram (not to scale!) for hydrogen: 12.8 1.00 atm 0.071 13.9 14.0 20.3 33.2 T Kelvin A sample of hydrogen at a pressure of 7.10x10 atm and a temperature of 11.4 K is compressed at constant temperature to a pressure of 18.2 atm. Which of the following are true? Choose all that apply O The liquid initially present will vaporize. O The final state of the substance is a solid. O The sample is initially a solid. O No phase change will occur. O The sample is initially a gas. Submit Answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY