
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
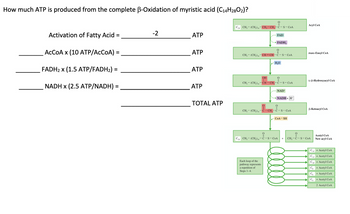
Transcribed Image Text:How much ATP is produced from the complete B-Oxidation of myristic acid (C14H2802)?
Activation of Fatty Acid
ACCOA x (10 ATP/ACCOA)
FADH₂ x (1.5 ATP/FADH₂) =
NADH x (2.5 ATP/NADH) :
=
=
-2
ATP
ATP
ATP
ATP
TOTAL ATP
C18 CH, (CH₂)₁4-CH₂-CH₂-C-S-COA
||
FAD
→FADH₂
CH₂(CH₂)₁4-CH=CH-C-S-CoA
Each loop of the
pathway represents
a repetition of
Steps 1-4.
H₂O
OH
CH₂(CH₂)₁4-CH-CH₂-C-S-CoA
||
- NAD+
→→NADH+ H
0
CH3-(CH₂) 14 C-CH₂-C-S-CoA
0
||
C16 CH₂(CH₂)14-C-S-CoA
COA-SH
Acyl CoA
trans-Enoyl COA
L-B-Hydroxyacyl CoA
B-Ketoacyl COA
H₁-C-S-
+ CH₂-C-S-CoA
Acetyl CoA
New acyl COA
C₁4+ Acetyl CoA
C12+ Acetyl CoA
CO+ Acetyl CoA
Cg+ Acetyl CoA
C
+ Acetyl COA
C₁ + Acetyl COA
2 Acetyl COA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Which of the following is higher during high levels of glutathione reductase and NADP? SH A но (GSH) OH NH2 NH2 HO OH ;-CH-CH2-CH2-C-NH-CH–ċ–NH–CH2- CH2 В (GSSG) CH2 -CH-CH;-CH2=C-NH–C NH-CH–Ç-NH-CH2-C HO OH NH2arrow_forward9. The equation below depicts the first step of the citric acid cycle. H0 CoA-SH CH-C + 0=C-CO0 HO C-COO citrate s-COA ČH2-COO synthase ČH2 -CO0 Citrate Acetyl-CoA Oxaloacetate AG" = -32.2 kJ/mol a) Explain the chemical conversions that take place during this step. b) Why is this reaction energetically favorable? Explain.arrow_forwardHelp me understand this…I’m confusedarrow_forward
- For saturated fatty acids, these reactions can continue as you have drawn until all the carbons of the fatty acid have been oxidized to acetyl-CoA. What is problematic about an unsaturated fatty acid, such as C16:cis-9?arrow_forwardCalculate the number of ATPATP generated from one saturated 1212‑carbon fatty acid. Assume that each NADHNADH molecule generates 2.5 ATP2.5 ATP and that each FADH2FADH2 molecule generates 1.5 ATP1.5 ATP .arrow_forwardPlease explain and give the correct answerarrow_forward
- The AG of the reaction C6H12O6 + 60₂ --> 6CO₂ + 6H₂O is -686 kcal/mol glucose oxidized. The AG of the reaction ADP + P₁ --> ATP + H₂O is + 7.3 kcal/mol ATP synthesized. The oxidation of glucose can be coupled to the synthesis of ATP. If the coupling is 50% efficient, how many molecules of ATP can be synthesized per molecule of glucose oxidized? Round your answer to the nearest whole number.arrow_forwardConsider the following reaction of the glycolysis pathway. Which statement is NOT true regarding this reaction? P + C-H NADH + H+ NAD+ C-0-P H-C-OH H-C-OH Glyceraldehyde- CH₂0-P 3-phosphate dehydrogenase CH₂0-P 1,3-Bisphosphoglycerate Glyceraldehyde-3- phosphate O A one-carbon compound (1C) is converted into a two-carbon compound (2C). O It requires a coenzyme. O It is a redox reaction catalyzed by the enzyme glyceraldehyde-3-phosphate dehydrogenase. O It is a phosphorylation reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON