
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
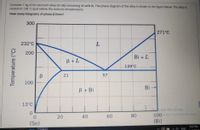
Transcribed Image Text:Consider 1 kg of tin-bismuth alloy (Sn-Bi) containing 40 wt% Bi. The phase diagram of the alloy is shown in the figure below. The alloy is
cooled to 138 "C (just below the eutectic temperature).
How many kilograms of phase B form?
300
271°C
232°C
200
Bi + L
В +L
139 C
21
100
Bi -
В + Bi
13°C
vate Windows
100gs to activate Windows
(Bi)
20
40
60
80
(Sn)
6:37 PM
A 4) ENG
57
B.
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements are true about the following phase diagram? Group of answer choices All three allotropes of iron exist at the same range of temperature and pressure. Iron vapor can exist at conditions of Earth's atmosphere. Solid iron can exist in three different crystal structures. Solid iron can exist in two different crystal structures. Liquid iron only exists at high pressure.arrow_forwardIn part 3 (polytropic) Why is T2 = 453.622 ?arrow_forward7. Consider 1.5 kg of a 99.6 wt% Fe-0.4 wt% C steel that is cooled to a temperature just below the eutectoid. (a) How many kilograms of ferrite (a) form? (b) How many kilograms of cementite (Fe;C) form? Include your tie line in the solution. 1600| L 1400 T(°C) 1200 (austenite) y+L 1148°C L+Fe;C 1000 Y + Fe;C 800 727°C 600 a + Fe;C 400 1 2 4 6 6.7 C, wt% C Fe;C (cementite)arrow_forward
- 1. four processes. 2. Fill out the blanks by labeling the phase regions. 3. Identify the Indicate an in- termediate compound, if any. 3. Temperature (°C) 1600 1400 1200 1000 800 600 400 1538°C 0 (Fe) -1493°C 1394°C 912°C y, Austenite 1 2 0.16 5 0,022 a, Ferrite A N 2.14 Composition (at% C) 15 10 3 1147°C L4 3 4 Composition (wt% C) 4.30 727°C Sketch the microstructure at A, label phases, figure out compositions. 20 Cementite (Fe3C). 5 6 25 6.70arrow_forwardA Pb-30% Sn alloy is cooled from 300°C to 50°C. a) What phases are present for this alloy at 50°C? b) How much of each phase is present at 50°C? c) What is the primary phase and its amount for this alloy at 50°C? Composition (at% Sn) 20 40 60 80 100 327°C 600 300 Liquid 500 232°C a + L 200 ß + L 400 183°C 18.3 61.9 97.8 300 100 a + B 200 100 40 60 80 100 (Pb) Composition (wt% Sn) (Sn) Temperature (°C) 20 Temperature (°F)arrow_forwardhelp pls A two-phase phase diagram is shown. Answer the following questions t -1300 1500+ Temperature 1100 0 34 54! 166 482 80 Composition of Ni % 20 What is the composition (in % Cu) of the solid phase corresponding to the red dot in between the solidus and liquidus lines in the phase diagram? Just write the number. Do not write the % symbol. Question 17 2.33 100 An alloy is being made with 66% Ni and 34% Cu. When it is cooled from 1500°C to the temperature corresponding to the red dot in the phase diagram, how many kg of liquid is present in the mixture?arrow_forward
- XCu versus temperature, liquid-solid phase- equilibrium diagram of a system consisting of Ag and Cu is given. a) Identify each zone and point O b) Define the temperatures T1, T2 and T3 c) Find the degrees of freedom for the points R, T, U, V and Y (f).arrow_forwardTemperature (°C) 700 600 0 500 400 300 0 (AI) a 5 a+L 10 Composition (at% Cu) 10 20 a +0 L 30 Composition (wt% Cu) 20 0+ L 0 (CuAl₂) 40 30 50 1200 1000 800 600 Temperature (°F) a) For the alloy represented by the phase diagram above, what is the wt% content of eutectic if an alloy of 2.5 wt% Cu is cooled very slowly from the molten state to 300°C? b) What phases are present in 2.5 wt% Cu material if it is quenched from 550°C?arrow_forwardQuestion 4 A segment of the Fe/C alloy phase diagram is shown below: Composition (at% C) 15 Temperature (°C) 0 1600 1400 1200 1000 800 600 400 1538°C 0 (Fe) 8 Y 912°C -1493 C 1394-C Y. Austenite 0.76 0.022 a, Ferrite 1 y+L 2 2.14 10 1147 C at Fe₂C L 4.30 Y+Fe3C 4 3 Composition (wt% C) 727°C 20 Cementite (Fe-C) 5 6 25 2500 2000 1500 1000 6.70 Temperature (°F) a) What are the equilibrium phases, compositions and relative fractions of the Fe-C alloy at an average composition Ccarbon of 2% and a temperature of 730 °C? b) What are the equilibrium phases, compositions and relative fractions of the Fe-C alloy at an average composition Ccarbon of 2% and a temperature of 725 °C? 7 c) Sketch the microstructure of the alloy obtained by quick cooling of the alloy from the conditions in part a) to the conditions in part b). Be sure to identify the phases in your sketch.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY