
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
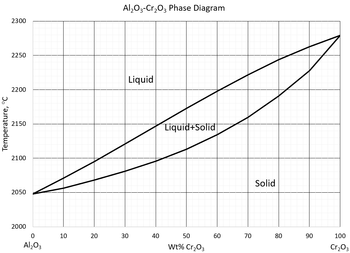
What is the composition, in wt% Cr2O3, of the liquid phase in an alloy containing 71 wt% Cr2O3 at 2200C?
Transcribed Image Text:Temperature, °C
2300
2250
2200
2150
2100
2050
2000
0
Al₂O3
10
20
Al2O3-Cr₂O3 Phase Diagram
Liquid
30
40
Liquid+Solid
50
Wt% Cr₂O3
60
70
Solid
80
90
100
Cr₂O3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 3. G T A A ४ L+d Liquid L L+α! d+d' al в Sotid G Liquid d B A For the eutectic phase diagram shown above at the temperature given by the dotted line, the two Gibbs free energy diagrams are equally valid. Explain the difference between the two and why they are both valid. ✓ Barrow_forwardFor alloys of two hypothetical metals A and B, there exist an x, A-rich phase, and a ß, B-rich phase. From the mass fractions of both phases for two different alloys provided in the table below, (which are at the same temperature), determine the composition of the phase boundary (solubility limit) for both a and ß phases at this temperature. Show complete solution. Alloy Composition Fraction & Phase Fraction 3 phase 70 wt% A - 30 wt% B 0.78 35 wt% A - 65 wt% B 0.36 0.22 0.64arrow_forwardQuenching is a process used to preserve at room temperature a phase which exists in equilibrium at high temperature but which would decompose during equilibrium cooling. Explain the basis of this process in the precipitation process and martensitic transformation. Use phase diagrams when necessary.arrow_forward
- It's not even that Idk how to do it, I'm very confused at that what parameters I should use to even begin answering. The initial masses of Ala dn Cu are 94.14g and 21.52g respectivelyarrow_forwardCould someone explain in detail how to solve this problem please. I really dont understand it. Thank you so much.arrow_forwardPlease explain carefullyarrow_forward
- BI (a) Figure BI below shows the equilibrium phase diagram of hypothetical A - B alloy system. Answer the following questions. What are technical names of Line A and Line B? What do they signify? (i) (ii) With the aid sketches, portray the microstructures and phases that are present for the 20wt%A-80wt%B alloy (Alloy A) at the temperatures of 780 °C, 670 °C, 652 °C, and 500 °C, respectively. (iii) Assume an A - B alloy (Alloy B) has 60wt%a - 40wt%L, in terms of weight, at a temperature of 652 °C. Determine the overall composition of Alloy B. (o) emai 700 8 600 500 400 800 0 A 760 °C Line B W a /9.5 20 Line A a + L 650 °C 40 Liquid (L) wt%B Figure B1 52.5 a + ß 60 B+L 80 93.7 B 100 Barrow_forwardExplain in detail how to apply the Clausius-Clapeyron equation to generate the phase diagram of a pure substance, explain differences between the different types of phase change.arrow_forwardNeed asaparrow_forward
- For alloys of two hypothetical metals A and B, there exists an a, A-rich phase and a b, B-rich phase. From the mass fractions of both phases for two different alloys (given below), which are at the same temperature, determine the composition of the phase boundary (or solubility limit) for the following: Alloy composition Fraction a Phase Fraction b Phase 60wt%A-40wt%B 0.60 0.40 30wt%A-70wt%B 0.12 0.88 a) a Phase = ___________ wt%A b) b Phase = ___________ wt%Aarrow_forward700 L 600 м a + L Mg, Pb 500 O 465 400 Mg Pb 300 200 a + Mg Pb B+ Mg,Pb 100 20 40 60 80 100 Mg Composition (wt% Pb) Pb Mg Pb Figure 1 The Mg/Pb phase diagram 1. The Pb% to gain alpha grain with Mg2Pb on the boundary of alpha at the room temperature. ANSWER: From the diagram, the required Pb% is 40% since it corresponds to the boundary eutectic. 2. Is it possible to gain beta grain with Mg2Pb on the boundary at the room temperature of an alloy? Explain ANSWER: It is not possible to get grain on Mg2Pb since the eutectic composition will be varied, which in turn will lose the composition strength. 3. What is the mount of alpha in an alloy (50%Mg +50%Pb) at temperature of 500oC? ANSWER: The mount of alpha in the alloy 50% Mg + 50 % Pb at 500 C will correspond to the eutectic composition at the 70% of Pb, i.e; the mount = 70/100 = 0.7 % of Pb 4. What is the mount of alpha in the alloy (50%Mg +50%Pb) at the room temperature? ANSWER: The mount of alpha in the alloy 50% Mg + 50 % Pb at room…arrow_forwardIf 20000 g of steam is condensed in 2 x 10^6 cm³ at a pressure of 4 MPa a. What is the temperature (C)? b. In what state steam? c. Draw it in the form of a phase diagram that you knowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY