
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
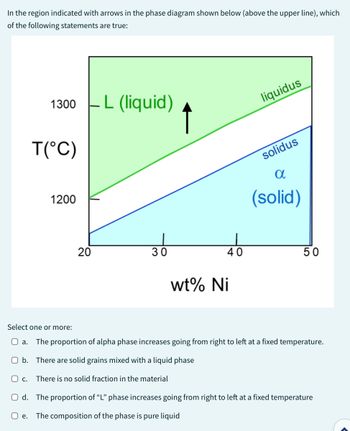
Transcribed Image Text:In the region indicated with arrows in the phase diagram shown below (above the upper line), which
of the following statements are true:
1300 - L (liquid)
O b.
O C.
T(°C)
1200
20
30
↑
wt% Ni
40
There are solid grains mixed with a liquid phase
There is no solid fraction in the material
liquidus
solidus
α
(solid)
Select one or more:
a. The proportion of alpha phase increases going from right to left at a fixed temperature.
50
Od. The proportion of "L" phase increases going from right to left at a fixed temperature
O e. The composition of the phase is pure liquid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- What is the composition, in wt% Cr2O3, of the liquid phase in an alloy containing 71 wt% Cr2O3 at 2200C?arrow_forwardXCu versus temperature, liquid-solid phase- equilibrium diagram of a system consisting of Ag and Cu is given. a) Identify each zone and point O b) Define the temperatures T1, T2 and T3 c) Find the degrees of freedom for the points R, T, U, V and Y (f).arrow_forwardTemperature (°C) 700 600 0 500 400 300 0 (AI) a 5 a+L 10 Composition (at% Cu) 10 20 a +0 L 30 Composition (wt% Cu) 20 0+ L 0 (CuAl₂) 40 30 50 1200 1000 800 600 Temperature (°F) a) For the alloy represented by the phase diagram above, what is the wt% content of eutectic if an alloy of 2.5 wt% Cu is cooled very slowly from the molten state to 300°C? b) What phases are present in 2.5 wt% Cu material if it is quenched from 550°C?arrow_forward
- b) Figure 1 shows a phase diagram for Cu-Ni binary system. Make a phase analysis of this system at point Q. T.°C 1450 1250 1050 0 20 40 60 80 100arrow_forwardThe point in the phase diagram where the fusion curve, the vapor pressure curve, and the sublimation curve join is called the Question 4 options: melting point. critical point. double point. boiling point. triple point. A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample? Question 5 options: It doubles. It halves. cannot be determined without more information. It quadruples. It does not change. Phase changes occur Question 6 options: as the temperature decreases. all of the above. none of the above. as the temperature remains the same. as…arrow_forwardShould correctarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY