
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
![Calculate the [OH-] and the pH of a solution with an [H] = 5.6 x 10-¹0 M at 25 °C.
[OH-] =
pH =
Calculate the [H*] and the pH of a solution with an [OH-] = 0.059 M at 25 °C.
pH =
Calculate the [H] and the [OH] of a solution with a pH = 2.70 at 25 °C.
[H*] =
M](https://content.bartleby.com/qna-images/question/50ee8c08-bdb6-49cd-9014-91cd3b523354/f2ee56a2-e119-4867-8570-55e8a3f324a9/qzr390s_thumbnail.jpeg)
Transcribed Image Text:Calculate the [OH-] and the pH of a solution with an [H] = 5.6 x 10-¹0 M at 25 °C.
[OH-] =
pH =
Calculate the [H*] and the pH of a solution with an [OH-] = 0.059 M at 25 °C.
pH =
Calculate the [H] and the [OH] of a solution with a pH = 2.70 at 25 °C.
[H*] =
M
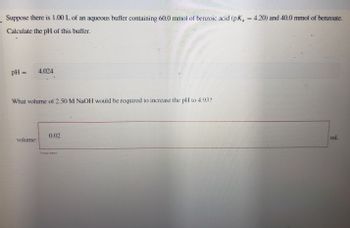
Transcribed Image Text:Suppose there is 1.00 L of an aqueous buffer containing 60.0 mmol of benzoic acid (pK₁ = 4.20) and 40.0 mmol of benzoate.
Calculate the pH of this buffer.
pH = 4.024
What volume of 2.50 M NaOH would be required to increase the pH to 4.932
0.02
volume:
ml.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- The reaction quotient is Q=1.6×10-26 Part B What pH is needed to produce this value of Q if the concentration and pressure values are [Br2]=2.50×10−4M , [Br−]=11.65M, [SO42−]=9.50M, and PSO2=3.50×10−5atm ? Express your answer numerically to two decimal places.arrow_forwardUsing your stock solution of 2 M glucose, you need to prepare the following series of glucose solutions for use in an experiment 0.8; 1.5, 1.75 and 2 M. Complete the table to show how you would dilute the sucrose to make 100ml of each of the solutions above.arrow_forwardConsider the following acids and their ionization constant, determine which conjugate base is HCOOH Ka = 1.7 x 10-4 (b) HCN Ka = 4.9 x 10-10arrow_forward
- A 2.0% (by mass) aqueous solution of novocainium chloride (C13 H21 CIN2O2) freezes at -0.235 °C. a Calculate the van't Hoff factor, i. - Kp (H2O) = -1.86 °C/m. i = b How many moles of ions are in the solution per mole of compound? mol ionsarrow_forwardPsilocybin is a natural product of psychotropic "magic" mushrooms and has the Following structure: HO HO O=0 Psilocybin The pK a Of the first phosphate oxygen is 1.3, the pK. Of the second phosphate oxygen is 6.2, And the pKa Of the tertiary amine is 10.4. What is the average charge of psilocybin when Dissolved in your carbonic acid buffer of pH=6?arrow_forwardHighlight your values of A,B,C and D. For your question: A mL of B mol/L sodium phosphate solution is combined with C mL of D mol/L calcium bicarbonate. (Calcium bicarbonate is soluble.) A mL B mol/L C mL D mol/L 75.0 ml 0.300 67.5 0.350 Before you begin your reaction, you must accurately produce 1.500 L of your sodium phosphate solution from sodium phosphate trihydrate solid. Write out a procedure to explain all the steps you will take in the lab when making the solution to ensure that your solution concentration is accurate. Please include calculations that show the required mass of solid. Also include the correct names of all equipment used.arrow_forward
- Nonearrow_forwardCalculate the pH of a solution if [H3O+] = 3.4 x 10-2M Indicate whether the solution is acidic, basic, or neutralarrow_forwardA monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H+ (aq) + A¯(aq) The equilibrium concentrations of the reactants and products are [HA] = 0.220 M, [H+] = 3.00 × 10−4 M, and [A¯] = 3.00 × 10−4 M. Calculate the value of pKa for the acid HA. pKa =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON