
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
What volume of 0.1M NaOH (reagent) needs to be added to increase your 7.5 pH (buffer) by 0.5 pH units. The goal is to further purify MOPs (see image).
Protonated form = 6.6 x 10.3^-3 mol/L
Deprotonated form = 1.32 x 10^-2 mol/L
Show calculations.
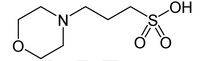
Transcribed Image Text:HO
N.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- B. You will need approximately 1 ml of the 100 µg/ml bromophenol blue (which is your most concentrated standard) to generate your standard curve. Calculate how much bromophenol blue stock solution and how much water you will combine to accomplish this. Make 1 ml of this solution using a 100-1000 μl micropipetter. Use the equation C₁V1=C2V2 μl of 1 mg/ml stock + μl of water = 1 ml of 100 µg/ml bromophenol bluearrow_forwardGel Running Buffer is made and kept at 14X concentration for storage. We will need 1.5 L of this solution at a concentration of 1X to run our gels. How would we make this solution? I am having trouble understanding the steps to this question, if you could please help me understand it that would be greatly appreciated!arrow_forwardWhat kind of buffer would you make with a optimal activity at ph 4.30?arrow_forward
- 80mL of a 0.3M solution of hexapeptide Leu-His-Cys-Glu-Asn-Arg is adjusted to pH=pI. The solution is then titrated with 0.2M HCL to a final pH of 2.1. Sketch the titration curve, labelling the pH and volume axes. Indicate the volume of HCL needed to reach relevant pKa value and equivalence point(s)z Relevant pKa values are: 2.1, 4.3, 6.0, 8.3, 9.8, and 12.5.arrow_forwardDescribe the preparation of 2.00 L of 0.100 M glycine buffer, pH 9.0, from glycine and 1.00 M NAOH. What mass of glycine is required, and what volume of 1.00 NaOH is required? The appropriate pK, of glycine is 9.6.arrow_forwardCalculate the pH of a buffer that contains 0.75 M acetic acid and 0.35 M acetate ion in 1 L solution. What will the pH of the buffer be upon the addition of 100.0 mL of 1.0 M NaOH? (Ka of acetic acid is 1.75 x 10-5 M)arrow_forward
- You have been provided with stock solutions of: stock A: 0.06 M sodium pyrophosphate buffer pH 8.5 stock B: 3 M ethanol stock C: 0.015 M NAD+ stock D: milli Q water Determine the volume you will need of each solution to prepare a buffer of with a final volume of 60 mL containing 10 mM sodium pyrophosphate pH 8.5, 100 mM ethanol, 1 mM NAD+. i.e. volume of stock A = _________mL volume of stock B = _________mL volume of stock C = _________mL volume of stock D = _________mL Show your calculations to arrive at your answers.arrow_forwardCalculate the volume of TAE buffer that you will need to prepare a 100ml solution of 1X strength if you only have a 50X concentrated solution available.arrow_forwardAcetic acid is the principal ingredient in vinegar as shown; that's why it tastes sour. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3 O+] = [CH3 CO2−] = 0.00118 M. What is the value of Ka for acetic acid?arrow_forward
- Identify the acid and conjugate base in each reaction. Calculate the pKa for each acid. List them in order from the strongest to weakest acid. The acid-ionization constants, Ka, at 25°C are listed for each. a. HC2H3O2 + H2O ↔ H3O+ + C2H3O2-acetic acid, KA = 1.7 x 10-5 b. HC7H5O2 + H2O H₂O+ + C7H5O2-benzoic acid, KA= 6.3 x 10-5 c. HC6H4NO2 + H2O ↔ H3O++ C6H4NO2-nicotinic acid, KA = 1.4 x 10-5arrow_forwardUsing your stock solution of 2 M glucose, you need to prepare the following series of glucose solutions for use in an experiment 0.8; 1.5, 1.75 and 2 M. Complete the table to show how you would dilute the sucrose to make 100ml of each of the solutions above.arrow_forwardCalculate the appropriate volume (in µL) of 9X loading buffer that should be added to 46.0 µL of a sample prior to loading the sample on an agarose gel.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON