
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
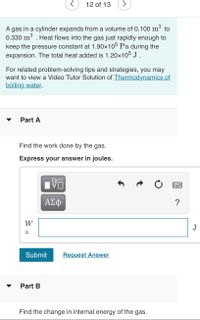
Transcribed Image Text:12 of 13
A gas in a cylinder expands from a volume of 0.100 m³ to
0.330 m . Heat flows into the gas just rapidly enough to
keep the pressure constant at 1.90x105 Pa during the
expansion. The total heat added is 1.20x105 J .
For related problem-solving tips and strategies, you may
want to view a Video Tutor Solution of Thermodynamics of
boiling water.
Part A
Find the work done by the gas.
Express your answer in joules.
?
W
J
Submit
Request Answer
Part B
Find the change in internal energy of the gas.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 2100 cm³ container holds 0.10 mol of helium gas at 350°C. Part A How much work must be done to compress the gas to 1200 Cm² at constant pressure? Express your answer with the appropriate units. ▸ View Available Hint(s) W = 220 J ✓ Correct Part B Previous Answers How much work must be done to compress the gas to 1200 Cm³ at constant temperature? Express your answer with the appropriate units. ► View Available Hint(s) W = Submit μA Value Previous Answers Units ? X Incorrect; Try Again; 4 attempts remainingarrow_forwardA crate of fruit with a mass of 40.0 kg and a specific heat capacity of 3550 J/(kg. K) slides 9.00 m down a ramp inclined at an angle of 40.0 degrees below the horizontal. Part A If the crate was at rest at the top of the incline and has a speed of 2.55 m/s at the bottom, how much work Wf was done on the crate by friction? Use 9.81 m/s² for the acceleration due to gravity and express your answer in joules. ► View Available Hint(s) Wf = -2140 J Submit Correct The frictional force opposes the motion of the crate, so the work done on the crate by friction must be a negative quantity. Part B Previous Answers If an amount of heat equal to the magnitude of the work done by friction is absorbed by the crate of fruit and the fruit reaches a uniform final temperature, what is its temperature change AT? ► View Available Hint(s) AT = ΑΣΦ Warrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Part A How much work is done by the gas in this process? Express your answer in joules. — ΑΣΦ W = Submit Part B On what is this work done? The work is done on the piston. The work is done on the cylinder. Submit Part C Request Answ AU = What is the change in internal energy of the gas? Express your answer in joules. ΑΣΦ Submit Request Answer Request Answer P Pearson W ? ? J Jarrow_forward
- Helparrow_forwardCan you please answer the follwing question, I did the right work but it seems that is not the right answer.arrow_forwardA heat engine does 160 J of work per cycle while exhausting 420 J of waste heat. Part A What is the engine's thermal efficiency? Express your answer as a percentage.arrow_forward
- A monatomic ideal gas is initially in state A. The gas undergoes a transition from state A by the three different processes shown, where process III is isothermal. In which process is energy added to the gas by heating? Explain briefly.arrow_forward5.0 g of nitrogen gas at 20°C and an initial pressure of 2.8 atm undergo a constant-pressure expansion until the volume has tripled. Part C How much heat is transferred to the gas to cause this expansion? Express your answer with the appropriate units. ► View Available Hint(s) Qexp 3000 J Submit Previous Answers ✓ Correct The gas pressure is then decreased at constant volume until the original temperature is reached. Part D What is the gas pressure after the decrease? Express your answer with the appropriate units. View Available Hint(s)arrow_forwardI am doing some practice problems to prepare for an exam and i cannot solve this problem, specifically part a but I would like to check this problem A piston cylinder device contains 0.8 kg of air initially at 100 kPa, 27 °C. The air is now compressed slowly in a polytropic process during which PV^1.3 =constant, until the volume reaches 1/2 its original volume. The system is then heated at constant pressure until the volume gets back to its original value. NOTE: THIS IS NOT A CYCLE. Data: Rair = 0.287 kJ/kg K; Cv=0.731 kJ/kg K; Cp=1.018 kJ/kgK. a) Determine the amounts AND directions of the Work and Heat(each in kJ) for each of the two processes. show all work used to get your values. b) Sketch the two processes on a P-v diagram, clearly showing all three state points and the two processes with their directions.arrow_forward
- An ideal gas at 20°C consists of 2.2 x 1022 atoms. 3.2 J of thermal energy are removed from the gas. Part A What is the new temperature in °C? Express your answer in degrees Celsius. DA ΑΣφ T = °C Submit Request Answerarrow_forwardpart b pleasearrow_forwardIn a Carnot engine the hot reservoir is 67.0°C warmer than the cold reservoir. The engine's efficiency is 17.5%. Part A What is the Kelvin temperature of the cold reservoir? Express your answer in kelvins. ? Tc = K Submit Request Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON