Question
part b please
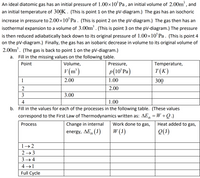
Transcribed Image Text:An ideal diatomic gas has an initial pressure of 1.00×10°Pa , an initial volume of 2.00m', and
an initial temperature of 300K. (This is point 1 on the pV-diagram.) The gas has an isochoric
increase in pressure to 2.00×10°P. . (This is point 2 on the pV-diagram.) The gas then has an
isothermal expansion to a volume of 3.00m'. (This is point 3 on the pV-diagram.) The pressure
is then reduced adiabatically back down to its original pressure of 1.00×10°P.. (This is point 4
on the pV-diagram.) Finally, the gas has an isobaric decrease in volume to its original volume of
2.00m. (The gas is back to point 1 on the pV-diagram.)
а.
Fill in the missing values on the following table.
Point
Volume,
Pressure,
Temperature,
v (m²)
p(10ʻPA)
T(K)
1
2.00
1.00
300
2
2.00
3
3.00
4
1.00
b. Fill in the values for each of the processes in the following table. (These values
correspond to the First Law of Thermodynamics written as: AE, =W +Q.)
Process
Change in internal
Work done to gas,
Heat added to gas,
energy, AE, (J)
W (3)
Q(1)
'th
1→ 2
2 → 3
3 → 4
4 →1
Full Cycle
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 7 steps with 7 images

Knowledge Booster
Similar questions
- A 65 kg person running at 2.0 m/s jumps onto the skim board at right. The skim board is 50 cm wide and 120 cm long, with an area of 0.60 m². Part A Draw a sketch of this situation. Part B If the person jumps onto the board on sand with a coefficient of friction of f=0.50, what force will resist the forward movement of the board [N]? Recall the friction force is the normal force times the coefficient of friction. Part C Now assume the person jumps onto the board on a 0.50 cm film of fresh water at 30°C. Use Newton's law of viscosity to estimate the force resisting the forward movement of the board.arrow_forwardTwo Blocks having mass m=11.0 kg are connected by a light string passing through two pulleys as shown. The block on the left is resting on an inclined plane of angle 0=45.0° . The pulleys and the planes on which the blocks are resting are frictionless. The blocks are released from rest and allowed to move freely. a. Draw the free body diamgrams for each block and then write down newton's second law of motion for each one separately. b. Find the acceleration of the blocks and the tension in the string.arrow_forwardhleparrow_forward
- d and e pleasearrow_forwardTwo ice skaters, with masses of 36.0 kg and 75.0 kg, are at the center of a 40.0 m diameter circular rink. The skaters push off against each other and glide to opposite edges of the rink. Part A If the heavier skater reaches the edge in 20.0 s , how long does the lighter skater take to reach the edge? ΑΣΦ Submit Request Answerarrow_forward
arrow_back_ios
arrow_forward_ios