
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
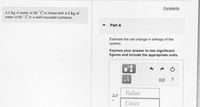
Transcribed Image Text:Constants
4.5 kg of water at 36 °C is mixed with 4.5 kg of
water at 60 ° C in a well-insulated container.
Part A
Estimate the net change in entropy of the
system.
Express your answer to two significant
figures and include the appropriate units.
HA
Value
AS
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Part C Since the aluminum bar is not an isolated system, the second law of thermodynamics cannot be applied to the bar alone. Rather, it should be applied to the bar in combination with its surroundings (the lake). Assume that the entropy change of the bar is -1238 J/K. What is the change in total entropy A.Stotal? Express your answer numerically in joules per Kelvin ▸ View Available Hint(s) AStotal = ΕΧΕΙ ΑΣΦ ? You have already submitted this answer. Enter a new answer. No credit lost. Try again. J/K Submit Previous Answersarrow_forwardPlease solve this question,Only if you have knowledge in this concept. Do not post random solutions.arrow_forward4.5 kg of water at 39 ^ °C is mixed with 4.5 kg of water at 67^°C in a well - insulated container. Part A Estimate the net change in entropy of the system. Express your answer to two significant figures and include the appropriate units.arrow_forward
- A Carnot engine performs work at the rate of 480 kW while using 930 kcal of heat per second. Part A If the temperature of the heat source is 590 °C, at what temperature is the waste heat exhausted? Express your answer using two significant figures. ? TŁ = °Carrow_forward2.0 mol of a monatomic ideal gas are at a temperature of 300 K and a pressure of 1.0 atm. What is the entropy change in a process that brings the gas to 350 K and 1.3 atm? Express your answer with the appropriate units.arrow_forwardA 4.0-kg piece of aluminum at 29.4 °C is placed in 1.0 kg of water in a Styrofoam container at room temperature (20.0 °C). Part A Estimate the net change in entropy of the system. Express your answer to two significant figures and include the appropriate units. AS = μA Value Units ?arrow_forward
- n moles of a diatomic ideal gas go through cycle a → b → c → d → a as shown in the figure. Processes ab and cd are isothermal and occur at temperatures TH and TC, respectively. 1) Calculate the work Wab done by the gas during the process a → b in terms of the variables in the problem. 2) Calculate the work Wbc done by the gas during the process b → c. 3) Calculate the total work W done in the entire cycle in terms of the variables in the problem. 4) Calculate the total heat Q flowing into the gas in a complete cycle.arrow_forwardplease do D and Earrow_forwardSix grams of helium (molecular mass = 4.0 u) expand isothermally at 370 K and does 9600 J of work. Assuming that the helium is an ideal gas, determine the ratio of the final volume of the gas to the initial volume. Number i Units Save for Later Attempts: 0 of 1 used Submit Answerarrow_forward
- Problem 1: Describe a situation in which the entropy of a container of gas is constant. In other words, come up with your own problem where the answer is that AS = 0.arrow_forwardCan you please answer the follwing question, I did the right work but it seems that is not the right answer.arrow_forwardOn a hot summer day, 2.00 x 10° J of heat transfer into a parked car takes place, increasing its temperature from 35.5°C to 44.4°C. What is the increase in entropy (in J/K) of the car due to this heat transfer alone? J/K Additional Materials CS Scanned with CamScanner O Readingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON