
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
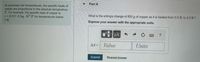
Transcribed Image Text:Part A
At extremely low temperatures, the specific heats of
metals are proportional to the absolute temperature
T. For example, the specific heat of copper is
c = (0.011 J/kg K)T for temperatures below
What is the entropy change of 850 g of copper as it is heated from 0.5 K to 2.0 K?
2 K.
Express your answer with the appropriate units.
HA
?
AS =
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- In an engine, an almost ideal gas is compressed adiabatically to half its volume. In doing so, 2530 J of work is done on the gas. Part A How much heat flows into or out of the gas? Express your answer with the appropriate units. ? Value Units %3D Submit Request Answer Part B What is the change in internal energy of the gas? Express your answer with the appropriate units. ? AU = Value Unitsarrow_forwardProblem 2 One mole of an ideal gas initially at 298 K and 100 k Pa is isothermally, reversibly compressed from 100 k Pa to 1000 k Pa. The internal energy U of an ideal gas only depends on temperature T and is independent of pressure p. For the process, please calculate the amount of each following quantity: a. Change in the internal energy of the ideal gas Au. b. Entropy produced A sir. c. Work done by the surrounding on the one mole of ideal gas. d. Heat transfer from the one mole of ideal gas to the surrounding. e. Entropy exchanged As between the one mole of ideal gas and the surrounding. f. Change in the molar entropy As of the one mole of ideal gas. g. Total entropy change A S,ot of the one mole of ideal gas plus the surrounding. h. Change in the mechanical energy of the one mole of ideal gas A Uy = – A (pv). i. Change in the thermal energy of the one mole of ideal gas Aur = A (Ts). j. Change in the chemical energy of the one mole of ideal gas Auc = Aµ. k. Change in the enthalpy…arrow_forwardYour calculator can't handle enormous exponents, but we can make sense of large powers of e by converting them to large powers of 10. If we write e = 10°, then eß = (10ª)³ = 108. What is the value of a? α = 0.43 Submit ✓ Correct Part B Previous Answers What is the multiplicity of a macrostate with entropy S = 0.80 J/K? Give your answer as a power of 10. IVE ΑΣΦ = 10; aß= 1.575 1023 Submit Previous Answers Request Answer X Incorrect; Try Again Provide Feedback ?arrow_forward
- Part C Since the aluminum bar is not an isolated system, the second law of thermodynamics cannot be applied to the bar alone. Rather, it should be applied to the bar in combination with its surroundings (the lake). Assume that the entropy change of the bar is -1238 J/K. What is the change in total entropy A.Stotal? Express your answer numerically in joules per Kelvin ▸ View Available Hint(s) AStotal = ΕΧΕΙ ΑΣΦ ? You have already submitted this answer. Enter a new answer. No credit lost. Try again. J/K Submit Previous Answersarrow_forward4.5 kg of water at 39 ^ °C is mixed with 4.5 kg of water at 67^°C in a well - insulated container. Part A Estimate the net change in entropy of the system. Express your answer to two significant figures and include the appropriate units.arrow_forwardA Carnot engine performs work at the rate of 480 kW while using 930 kcal of heat per second. Part A If the temperature of the heat source is 590 °C, at what temperature is the waste heat exhausted? Express your answer using two significant figures. ? TŁ = °Carrow_forward
- Part A Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from P₁ = 2.7 atm to P2 = 1.7 atm. Then the gas expands at constant pressure, from a volume of V₁ = 5.9 L to V₂ = 9.6 L, where the temperature reaches its original value. See the figure ( Figure 1). Calculate the total work done by the gas in the process. Express your answer to two significant figures and include the appropriate units. ☐ о W = Value ? Units Figure P P₁ b P2 P 1 of 1 Submit Request Answer Part B Calculate the change in internal energy of the gas in the process. Express your answer with the appropriate units. με ? AU = Value Units V₁ V2 Varrow_forwardplease answer all parts they are related. A) What is the thermal efficiency of this engine B) what is the total change in entropy of this ideal gas after after completing the engine cycle?arrow_forwardPart A A gas is compressed from 600 cm to 200 cm³ at a constant pressure of 500 kPa. At the same time, 100 J of heat energy is transferred out of the gas. What is the change in thermal energy of the gas during this process? For general problem-solving tips and strategies for this topic, you may want to view a Video Tutor Solution of Fire piston. Express your answer with the appropriate units. μΑ AEth Value Jarrow_forward
- A 4.0-kg piece of aluminum at 29.4 °C is placed in 1.0 kg of water in a Styrofoam container at room temperature (20.0 °C). Part A Estimate the net change in entropy of the system. Express your answer to two significant figures and include the appropriate units. AS = μA Value Units ?arrow_forwardplease do D and Earrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Part A How much work is done by the gas in this process? Express your answer in joules. — ΑΣΦ W = Submit Part B On what is this work done? The work is done on the piston. The work is done on the cylinder. Submit Part C Request Answ AU = What is the change in internal energy of the gas? Express your answer in joules. ΑΣΦ Submit Request Answer Request Answer P Pearson W ? ? J Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON