
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
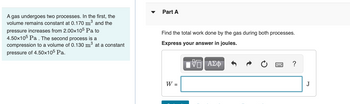
Transcribed Image Text:A gas undergoes two processes. In the first, the
volume remains constant at 0.170 m³ and the
pressure increases from 2.00x105 Pa to
4.50x105 Pa. The second process is a
compression to a volume of 0.130 m³ at a constant
pressure of 4.50x105 Pa.
Part A
Find the total work done by the gas during both processes.
Express your answer in joules.
W =
VE ΑΣΦ
?
J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Part A How much work is done by the gas in this process? Express your answer in joules. — ΑΣΦ W = Submit Part B On what is this work done? The work is done on the piston. The work is done on the cylinder. Submit Part C Request Answ AU = What is the change in internal energy of the gas? Express your answer in joules. ΑΣΦ Submit Request Answer Request Answer P Pearson W ? ? J Jarrow_forwardA heat engine does 30.0 J of work and exhausts 35.0 J of waste heat during each cycle. Part A What is the engine's thermal efficiency? Express your answer as a percentage. 46.2 % Submit Correct Part B Previous Answers If the cold-reservoir temperature is 30.0 °C, what is the minimum possible temperature in °C of the hot reservoir? Express your answer in degrees Celsius. —| ΑΣΦ 383.35 ? °Carrow_forwardA large cylindrical tank contains 0.750 m³ of nitrogen gas at 22.0°C and 6.50x103 Pa (absolute pressure). The tank has a tight-fitting piston that allows the volume to be changed. Part A What will be the pressure if the volume is decreased to 0.530 m³ and the temperature is increased to 165°C? Express your answer in pascals. —| ΑΣΦ P = Submit Request Answer < Return to Assignment Provide Feedback ? Paarrow_forward
- The heat engine shown in the figure uses 2.0 mol of a monatomic gas as the working substance. (Figure 1) Figure p (kPa) 600 400 200 0 0 0.025 0.050 < 1 of 1 V (m³) Part D Determine AEth, Ws, and Q for 3→1. Enter your answers numerically separated by commas. Express your answer using two significant figures. VE ΑΣΦ AEth, Ws, Q = Submit Part E Request Answer What is the engine's thermal efficiency? Express your answer using two significant figures. ? Jarrow_forwardIn an engine, an almost ideal gas is compressed adiabatically to half its volume. In doing so, 2710 J of work is done on the gas. Part A ▼ How much heat flows into or out of the gas? Express your answer with the appropriate units. ? Q = Value Units Submit Request Answer Part B What is the change in internal energy of the gas? Express your answer with the appropriate units. HẢ ? AU = Value Units Submit Request Answerarrow_forward4.5 mol of monatomic gas A interacts with 2.9 mol of monatomic gas B. Gas A initially has 9000 J of thermal energy, but in the process of coming to thermal equilibrium it transfers 800 J of heat energy to gas B. How much thermal energy did gas B have initially? Express your answer with the appropriate units. ► View Available Hint(s) EBi = Submit Value 4 Previous Answers Units ?arrow_forward
- Can you help with "c" was told to use as less digits as possible 3. An unknown number of moles of an ideal monoatomic gas expand reversibly from Vi = 3.10 m3 to Vf = 4.00 m3, at a constant pressure of 1.47 atm and an initial temperature of 300 K. a. Find the number of moles of gas. 185b. Find the final temperature of the gas K. 387 c. Calculate the work done by the gas. 134053 J <<<< this is the wrong answer. (should use less digits for this answer)arrow_forwardA cylinder contains 0.250 mol of carbon dioxide (CO₂) gas at a temperature of 27.0°C. The cylinder is provided with a frictionless piston, which maintains a constant pressure of 1.00 atm on the gas. The gas is heated until its temperature increases to 127.0°C. Assume that the CO₂ may be treated as an ideal gas. Express your answer in joules. AU = Submit Part D Submit How much heat was supplied to the gas? Express your answer in joules. Part E W₁ = ΑΣΦ | Submit Request Answer ΑΣΦ Request Answer Request Answer < Return to Assignment Provide Feedback www How much work would have been done if the pressure had been 0.50 atm? Express your answer in joules. VE ΑΣΦ P Pearson = ? ? J ? J Jarrow_forwardCan You please answer the following by breaking down the working.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON