
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
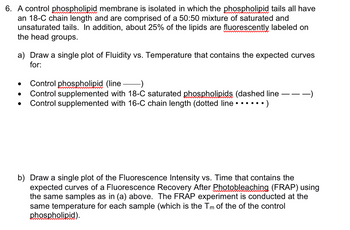
Transcribed Image Text:6. A control phospholipid membrane is isolated in which the phospholipid tails all have
an 18-C chain length and are comprised of a 50:50 mixture of saturated and
unsaturated tails. In addition, about 25% of the lipids are fluorescently labeled on
the head groups.
a) Draw a single plot of Fluidity vs. Temperature that contains the expected curves
for:
Control phospholipid (line- -)
Control supplemented with 18-C saturated phospholipids (dashed line -
Control supplemented with 16-C chain length (dotted line • • • • • • )
b) Draw a single plot of the Fluorescence Intensity vs. Time that contains the
expected curves of a Fluorescence Recovery After Photobleaching (FRAP) using
the same samples as in (a) above. The FRAP experiment is conducted at the
same temperature for each sample (which is the Tm of the of the control
phospholipid).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Consider a mixture comprised of the proteins attached: Protein that will most strongly bind to an anion exchange column? Protein that will elute first in gel filtration chromatography? Protein that will elute last in hydrophobic interaction chromatography? Protein that will elute last in carbohydrate containing column?arrow_forward1. Complete the table below with information about the amino acids utilized in this pro- cedure. Remember that smaller amino acids will travel further and so will amino acids that are soluble in the solvent. Table 9-1. Amino Acids Procedure AMINO ACIDS Phenylalanine Aspartic Acid Leucine Proline matemps DRAW THE MOLECULAR STRUCTURE in POLARITY (IS IT POLAR OR NONPOLAR?) odme her basalu la MOLECULAR WEIGHT SIZE (RANK LARGEST = 4 AND SMALLEST = 1) SHOULD IT REACT WITH NINHYDRIN?arrow_forwardIt seems paradoxical that a lipid bilayer can be fluid yet asymmetrical. Explain.arrow_forward
- (c) The tube and cylinder diagram to the right illus- trates schematically the potassium channel protein from a bacterium. This channel protein consists of four identical polypeptide chains each comprised of 119 residues. The cyan ribbon illustrates only two of the polypeptide chains. The relative positions of 4 α-helices, one from each poly- peptide chain, are shown as cylinders. The red sphere in the central part of the diagram shows the position of a potas- sium ion identified by X-ray structure analysis. A cesium ion (green sphere) was also found to be stabilized in the same position when cesium chloride was introduced into the crys- tals. The cylinders each represent an α-helix running from Tyr62 to Thr74. Considering the position of the potassium ion, in- dicate on one of the cylinders at which end of the cylinder residue Tyr62 is found and at which end residue Thr74 is found. Explain the basis of your answer? Ala23 Thr119 (d) The N- and C-terminal residues of one of the…arrow_forward2. | Calculate the overall charge (pH 7) on the following three polypeptides and answer the questions below. Assume the following pKa values: N-terminal -NH3®, 7.0; all -COOH groups, 4.0; Arg, 12.5; Cys, 8.4; His, 6.0; Lys, 10.0; Tyr, 10.0. (A) Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val-Lys-Val-Tyr-Pro-Asp-Ala -Gly- Glu-Asp-Gln- Ser-Ala-Glu-Ala-Phe-Pro-Leu-Arg-Glu-Phe (B) Ser-Tyr-Ser-Met-Glu–His-Phe-Arg–Trp–Gly-Ala-Pro-Val-Gly-Glu-Glu-Cys-Asp-Pro-Val-Glu–Val-Tyr-Pro-Asp- Ala-Gly-Glu-Asp-Gln-Ser-Ala-Glu-Ala-Phe-Pro-Leu-Glu-Phe-Cys-Ser-Tyr-Ser-Met-Glu-His-Phe-Asp-Trp-Gly- Asp-Pro-Val-Gly-Pro-Asp-Ala-Gly-Asp-Gln-Pro-Val-Gly-Glu-Glu-Cys-Asp-Pro-Val-Glu-Val-Tyr-Pro-Asp-Ala | (C) Gly-Ser-Val-Arg-Asp-Pro-Val-Lys-Glu–Val-Tyr-Pro-Asp- Lys-Ala-Gly-Arg-Glu-Ser-Arg-Ala (a) Which of the three peptides would elute first from a gel filtration column? (b) Which of the three peptides would migrate the fastest on SDS-PAGE (c) Which of the three peptides could be…arrow_forward4. A protein solution is prepared by dissolving 350 µg of protein in 200 µL of water. A 150 μL sample of this solution is diluted to a total volume of 4.5 mL. How many mg of protein will be in a 2 mL sample of the diluted protein solution? Space to show your workings:arrow_forward
- 3. You have a protein that is easily extracted from a cell membrane preparation, without any associated lipids, by washing with a buffer containing a salt (e.g. 2M NaBr) without the need to treat the membrane with detergents. What type of protein do you have? E) Peripheral (extrinsic) F) A protein covalently anchored to membrane fatty acids G) Transmembrane H) None of the above Answer: Explanation: 4. A bacterium is suddenly expelled from a warm human intestine into the cold world outside (hey, it's been a cold December!). Which of the following adjustments might the bacterium make to maintain the same level of membrane fluidity? I) Produce lipids with hydrocarbon tails that are longer and have fewer double bonds Produce lipids with hydrocarbon tails that are shorter and have more double bonds K) Decrease the amount of cholesterol in the membrane L)Decrease the amount of glycolipids in the membrane Answer: Explanation: 5. Which of the following membrane lipids does not contain fatty…arrow_forwardWhen the potato cube is surrounded by a hypotonic solution what change in mass occurs in the potato cells?arrow_forwardOf the following cell membrane lipids, which one prefers to reside in the inner leaflet (or inner half) of the membrane bilayer, AND has an overall neutral charge at physiological pH (7.4)? a) PE (phosphatidylethanolamine) is inner-leaflet with an overall neutral charge at pH 7.4 b) SM (sphinogomyelin) is inner-leaflet with an overall neutral charge at pH 7.4 c) PS (phosephatidylserine) is inner-leaflet with an overall neutral charge at pH 7.4 d) PC (phosphatidylcholine) is inner-leaflet with an overall neutral charge at pH 7.4arrow_forward
- 2. | Calculate the overall charge (pH 7) on the following three polypeptides and answer the questions below. Assume the following pKa values: N-terminal –NH3®, 7.0; all -COOH groups, 4.0; Arg, 12.5; Cys, 8.4; His, 6.0; Lys, 10.0; Tyr, 10.0. (A) Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys–Pro–Val–Gly–Lys–Lys–Arg-Arg-Pro-Val-Lys–Val-Tyr-Pro-Asp-Ala -Gly- Glu-Asp-GIn– Ser-Ala-Glu-Ala-Phe-Pro-Leu-Arg-Glu-Phe (B) Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Ala-Pro-Val-Gly-Glu-Glu–Cys-Asp-Pro-Val-Glu–Val–Tyr-Pro-Asp- Ala-Gly-Glu-Asp-Gln-Ser-Ala-Glu-Ala-Phe-Pro-Leu-Glu-Phe-Cys-Ser-Tyr-Ser-Met-Glu–His-Phe-Asp-Trp-Gly- Asp-Pro-Val-Gly-Pro-Asp-Ala-Gly-Asp-Gln-Pro-Val–Gly–Glu-Glu-Cys-Asp-Pro–Val-Glu–Val–Tyr-Pro-Asp-Ala (C) Gly-Ser-Val-Arg-Asp-Pro-Val-Lys-Glu-Val-Tyr-Pro-Asp- Lys–Ala-Gly-Arg-Glu-Ser-Arg-Ala (d) Which of the three peptides would migrate the closest to the anode in isoelectric focusing? (e) Which of the above peptides would elute last from a gel filtration column? (f) Which of the three…arrow_forward20. The dissociation constant of protein Z for ligand Y is: 10 micromolar. What fraction of the ligand is bound at ligand concentration = 5 micromolar?arrow_forwardExplainarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON