
Concept explainers
Bromination of 2-methylbutane yields predominantly one product with the formula
(a) 2-Methyl-2-butene
(b) 2-Methyl-2-butanol
(c) 3-Methyl-2-butanol
(d) 3-Methyl-1-butyne
(e) 1-Bromo-3-methylbutane
(f) 2-Chloro-3-methylbutane
(g) 2-Chloro-2-methylbutane
(h) 1-Iodo-3-methylbutane
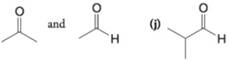
(i)
Want to see the full answer?
Check out a sample textbook solution
Chapter FRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Fundamentals of Physics Extended
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: Structure and Properties (2nd Edition)
Microbiology: An Introduction
Microbiology: An Introduction
Principles of Anatomy and Physiology
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

