
(a)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the
Addition of water to the given starting material creates bifunctional compound.
(b)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
(c)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
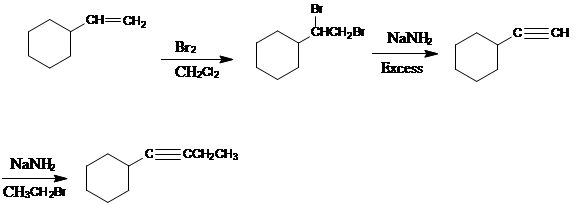 Adding
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
(d)
Interpretation:
A multi-step synthesis has to be designed for the given conversion.
Concept Introduction:
Target molecule is nothing but the desired product.
Adding
The elimination reaction should be carried out under SN2 condition i.e. with strong nucleophile. So there will be no compensating substitution reaction.
The bulky base is used in the elimination reaction to maximize the amount of elimination product.
To prepare cyclic ether, the alkyl halide and alcohol must be a part of the same molecule.
Addition of water to the given starting material creates bifunctional compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry (8th Edition)
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
