
(a)
Interpretation:
Which one is a better nucleophiles has to be identified.
Concept introduction:
Nucleophilicity is a measure of how readily a compound is able to attack an electron-deficient atom.
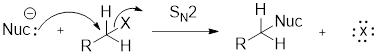
Structure of the substrate plays major role in the reactivity of
(b)
Interpretation:
Which one is a strong base has to be identified.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Essential Organic Chemistry, Global Edition
- Rank the species in each group in order of increasing nucleophilicity. a. CH,, "OH, "NH2 b. H20, OH, "SH in CH3OH c. CH,CH2S", CH;CH2O¯, CH;COO" in CH3OH d. CH3NH2, CH3SH, CH;OH in acetone e. "OH, F", CI" in acetone f. HS, F", CI in CH3OHarrow_forwardIdentify the stronger nucleophile in each pair. a. NH3,−NH2 b. CH3NH2, CH3OH c. CH3CO2−, CH3CH2O−arrow_forwardIdentify the stronger nucleophile in each pair. a.NH3, −NH2 b.CH3NH2, CH3OH c.CH3CO2−, CH3CH2O−arrow_forward
- Rank the species in each group in order of increasing nucleophilicity. a. CH3CH2S−CH3CH2O−, CH3CO2− in CH3OH b. CH3NH2, CH3SH, CHOH in acetone c.−OH, F−, Cl− in acetone d. HS−, F−, Cl− in CH3OHarrow_forwardIdentify the stronger nucleophile in each pair of anions. a.Br− or Cl− in a polar protic solvent b.HO− or Cl− in a polar aprotic solvent c.HS− or F− in a polar protic solventarrow_forwardWhich is a better nucleophile? a. Br or C in H20 b. Br or CI in DMSO c. CH3O or CH;OH in H20 d. CH30 or CH3OH in DMSO e. HO or NH2 in H20 f. HO or NH, in DMSO g. F or Br in H20 h. I or Br in DMSOarrow_forward
- Rank the species in each group in order of increasing nucleophilicity.a. CH3CH2S-, CH3CH2O-, CH3CO2- in CH3OHb. CH3NH2, CH3SH, CH3OH in acetonec. -OH, F-, Cl- in acetoned. HS-, F-, Cl- in CH3OHarrow_forward1. Draw the products of each nucleophilic substitution reaction a. b. D b OH C. d. e. f. Br 1 NaCN + NaOCH3 H₂Oarrow_forwardDraw the substitution product that results when CH,CH2CH;CH,Br reacts with each nucleophile. d. "OCH(CH3)2 e. "C=CH f. H20 a. "OH g. NH3 b. "SH h. Nal c. "ČN i. NaNgarrow_forward
- Which member in each pair is a better leaving group? a. H2O or HO- b. NH3 or H2O c. H2O or H2S d. HO- or HS- e. I- or Br-. f. Cl- or Brarrow_forwardIn which category does NaNHCH2CH3 belong? A. Good nucleophile, weak base. В. B. Good nucleophile, strong base. Poor nucleophile, weak base. Poor nucleophile, strong base.arrow_forwardWhich SN1 reaction is each pair is faster? А. CH,OH CH;OH B. Br CH CHOН eBr OMSO Br CH,CH OH CH,CH,OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





