
Biology
5th Edition
ISBN: 9781260487947
Author: BROOKER
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7.6, Problem 2EQ
CoreSKILL » In the experiment of Figure 7.13, what observation indicated to the researchers that ATP synthase is a rotary machine? What was the control of this experiment? What did it indicate?
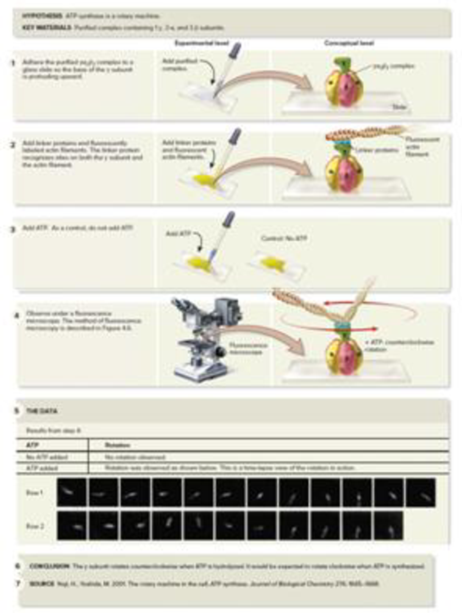
Figure 7.13 Yoshida and Kinosita provide evidence that ATP synthase is a rotary machine.
(5): From Noji, H., Yoshida, M. 2001. The Rotary Machine in the Cell, ATP Synthase. Journal of Biological Chemistry 276: 1665–1668. ©2001 The American Society for Biochemistry and Molecular Biology
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please help thank you
How are sharks different than all the other species
Answer number seven do what it says.
Chapter 7 Solutions
Biology
Ch. 7.2 - Core Skill: Connections Look ahead to Table 45.1....Ch. 7.2 - Which organic molecules donate a phosphate group...Ch. 7.2 - Prob. 2CSCh. 7.4 - Prob. 1CCCh. 7.5 - Explain the meaning of the name cytochrome...Ch. 7.6 - Prob. 1CCCh. 7.6 - Prob. 1CSCh. 7.6 - Prob. 1EQCh. 7.6 - CoreSKILL In the experiment of Figure 7.13, what...Ch. 7.6 - Prob. 3EQ
Ch. 7.7 - Prob. 1CCCh. 7 - Which of the following pathways occurs in the...Ch. 7 - Prob. 2TYCh. 7 - Prob. 3TYCh. 7 - Which organic molecule supplies a two-carbon group...Ch. 7 - The ability to diagnose tumors using...Ch. 7 - Prob. 6TYCh. 7 - Certain drugs, which are called ionophores, cause...Ch. 7 - Prob. 8TYCh. 7 - Prob. 9TYCh. 7 - Prob. 10TYCh. 7 - Prob. 1CQCh. 7 - What causes the rotation of the subunit of ATP...Ch. 7 - Core Concept: Energy and Matter How is glucose...Ch. 7 - Discuss the advantages and disadvantages of...Ch. 7 - Prob. 2COQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the process that is "capable of destroying all forms of microbial life"? Question 37 options: Surgical scrub Sterilization Chemical removal Mechanical removalarrow_forwardAfter you feel comfortable with your counting method and identifying cells in the various stages of mitosis, use the four images below of whitefish blastula to count the cells in each stage until you reach 100 total cells, recording your data below in Data Table 1. (You may not need to use all four images. Stop counting when you reach 100 total cells.) After totaling the cells in each stage, calculate the percent of cells in each stage. (Divide total of stage by overall total of 100 and then multiply by 100 to obtain percentage.) Data Table 1Stage Totals PercentInterphase Mitosis: Prophase Metaphase Anaphase Telophase Cytokinesis Totals 100 100% To find the length of time whitefish blastula cells spend in each stage, multiply the percent (recorded as a decimal, in other words take the percent number and divide by 100) by 24 hours. (Example: If percent is 20%, then Time in Hours = .2 * 24 = 4.8) Record your data in Data…arrow_forwardWhat are Clathrin coated vesicles and what is their function?arrow_forward
- How is a protein destined for the Endoplasmic Reticulum (ER), imported into the ER? Be concise.arrow_forwardFind out about the organisations and the movements aimed at the conservation of our natural resources. Eg Chipko movement and Greenpeace. Make a project report on such an organisation.arrow_forwardWhat are biofertilizers and mention the significancearrow_forward
- PCBs and River Otters: Otters in Washington State’s Green-Duwamish River have high levels of polychlorinated biphenyls (PCBs) in their livers. PCBs can bind to the estrogen receptors in animals and disrupt the endocrine system of these otters. The PCBs seem to increase the estrogen to androgen ratio, skewing the ratio toward too much estrogen. How would increased estrogen affect the river otter population? Based on your reading of the materials in this unit, what factors can affect fertility in humans? Explain how each of the factors affecting human fertility that you described can disrupt the human endocrine system to affect reproduction.arrow_forwardOther than oil and alcohol, are there other liquids you could compare to water (that are liquid at room temperature)? How is water unique compared to these other liquids? What follow-up experiment would you like to do, and how would you relate it to your life?arrow_forwardSelection of Traits What adaptations do scavengers have for locating and feeding on prey? What adaptations do predators have for capturing and consuming prey?arrow_forward
- Competition Between Species What natural processes limit populations from growing too large? What are some resources organisms can compete over in their natural habitat?arrow_forwardSpecies Interactions Explain how predators, prey and scavengers interact. Explain whether predators and scavengers are necessary or beneficial for an ecosystem.arrow_forwardmagine that you are conducting research on fruit type and seed dispersal. You submitted a paper to a peer-reviewed journal that addresses the factors that impact fruit type and seed dispersal mechanisms in plants of Central America. The editor of the journal communicates that your paper may be published if you make ‘minor revisions’ to the document. Describe two characteristics that you would expect in seeds that are dispersed by the wind. Contrast this with what you would expect for seeds that are gathered, buried or eaten by animals, and explain why they are different. (Editor’s note: Providing this information in your discussion will help readers to consider the significance of the research).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College


Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license