
(a)
Interpretation:
The rate for the given reaction should be analyzed for the given conditions.
Concept Introduction:
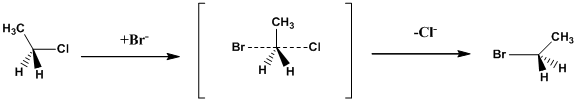
Structure of the substrate plays a major role in
Second-Order rate reaction: It is a type of
(b)
Interpretation:
The rate for the given reaction should be analyzed for the given conditions.
Concept Introduction:

Structure of the substrate plays a major role in
Second-Order rate reaction: It is a type of chemical reaction in which the reaction depends on concentration of one second order reactant or both the first order reactants involved in the reaction.
(c)
Interpretation:
The rate for the given reaction should be analyzed for the given conditions.
Concept Introduction:

Structure of the substrate plays a major role in
Second-Order rate reaction: It is a type of chemical reaction in which the reaction depends on concentration of one second order reactant or both the first order reactants involved in the reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry
- Show work with explanation needed. Don't give Ai generated solutionarrow_forward7. Calculate the following for a 1.50 M Ca(OH)2 solution. a. The concentration of hydroxide, [OH-] b. The concentration of hydronium, [H3O+] c. The pOH d. The pHarrow_forwardA first order reaction is 46.0% complete at the end of 59.0 minutes. What is the value of k? What is the half-life for this reaction? HOW DO WE GET THERE? The integrated rate law will be used to determine the value of k. In [A] [A]。 = = -kt What is the value of [A] [A]。 when the reaction is 46.0% complete?arrow_forward
- 3. Provide the missing compounds or reagents. 1. H,NNH КОН 4 EN MN. 1. HBUCK = 8 хно Panely prowseful kanti-chuprccant fad, winddively, can lead to the crading of deduc din-willed, tica, The that chemooices in redimi Грин. " like (for alongan Ridovi MN نيا . 2. Cl -BuO 1. NUH 2.A A -BuOK THE CF,00,H Ex 5)arrow_forward2. Write a complete mechanism for the reaction shown below. NaOCH LOCH₁ O₂N NO2 CH₂OH, 20 °C O₂N NO2arrow_forward4. Propose a synthesis of the target molecules from the respective starting materials. a) b) LUCH C Br OHarrow_forward
- The following mechanism for the gas phase reaction of H2 and ICI that is consistent with the observed rate law is: step 1 step 2 slow: H2(g) +ICI(g) → HCl(g) + HI(g) fast: ICI(g) + HI(g) → HCl(g) + |2(g) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. + → + (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form k[A][B]"..., where '1' is understood (so don't write it) for m, n etc.) Rate =arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward1. For each of the following statements, indicate whether they are true of false. ⚫ the terms primary, secondary and tertiary have different meanings when applied to amines than they do when applied to alcohols. • a tertiary amine is one that is bonded to a tertiary carbon atom (one with three C atoms bonded to it). • simple five-membered heteroaromatic compounds (e.g. pyrrole) are typically more electron rich than benzene. ⚫ simple six-membered heteroaromatic compounds (e.g. pyridine) are typically more electron rich than benzene. • pyrrole is very weakly basic because protonation anywhere on the ring disrupts the aromaticity. • thiophene is more reactive than benzene toward electrophilic aromatic substitution. • pyridine is more reactive than nitrobenzene toward electrophilic aromatic substitution. • the lone pair on the nitrogen atom of pyridine is part of the pi system.arrow_forward
- The following reactions are NOT ordered in the way in which they occur. Reaction 1 PhO-OPh Reaction 2 Ph-O -CH₂ heat 2 *OPh Pho -CH2 Reaction 3 Ph-O ⚫OPh + -CH₂ Reaction 4 Pho Pho + H₂C OPh + CHOPh H₂C -CH₂ Reactions 1 and 3 Reaction 2 O Reaction 3 ○ Reactions 3 and 4 ○ Reactions 1 and 2 Reaction 4 ○ Reaction 1arrow_forwardSelect all possible products from the following reaction: NaOH H₂O a) b) ОН HO O HO HO e) ОН f) O HO g) h) + OHarrow_forward3. Draw diagrams to represent the conjugation in these molecules. Draw two types of diagram: a. Show curly arrows linking at least two different ways of representing the molecule b. Indicate with dotted lines and partial charges (where necessary) the partial double bond (and charge) distribution H₂N* H₂N -NH2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





