
Interpretation:
The major product for the given reaction conditions should be identified.
Concept Introduction
Structure of the substrate plays major role in the reactivity of
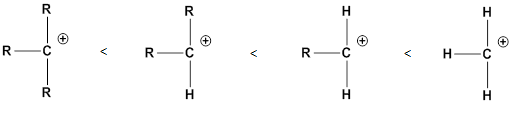
Leaving group: it is a fragment that leaves substrate with a pair of electrons via heterolytic bond cleavage.
Nucleophile: donates pair of electrons to positively charged substrate resulting in the formation of
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry
- Show work with explanation needed..don't give Ai generated solutionarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardIn the box on the right, draw the best resonance structure of the compound on the left. Draw electron-flow arrows on the structure on the left to indicate how the electrons reorganize to give the structure on the right. Interactive 3D display mode CH₁₂ Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. CONT を口か H3C. CH3 H C Zo S CI Br P9 Farrow_forward
- identify which of the following pairs of amino acids residues can have London dispersion forces between their side chains. a. Alanine and Glycine b. Leuccine and Isoleucin c. Valine and Asparagine d. Threonine and Tyrosinearrow_forwardShow work with explanation needed..don't give Ai generated solutionarrow_forwardGive detailed Solution with explanation needed...don't give Ai generated solutionarrow_forward
- Show work.....don't give Ai generated solutionarrow_forwardDraw the organic product(s) of the following reaction. CH3 CH3 NBS monosubstitution products CCl4 You do not have to consider stereochemistry. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





