
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.34P
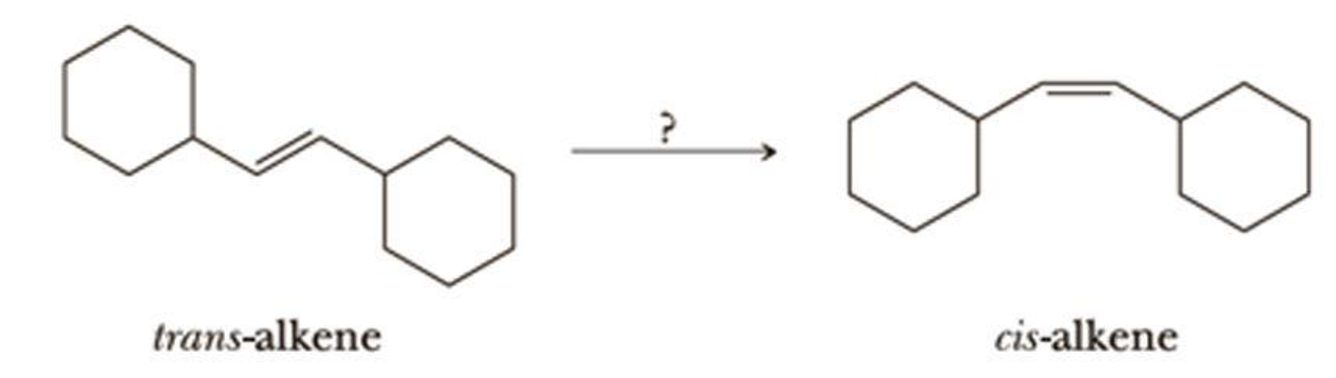
Using your reaction roadmap as a guide, show how to convert the starting trans-
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
draw the two possible carbocations that can form when this alkene reacts with a strong acid (such as HBr or H3O+). of the two structures you drew, circle the more stable carbocation
H3C
CH3
H3C
NA C→XT
Br
Br₂
CH₂Cl₂
H3C
Electrophilic addition of bromine, Br₂, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic
intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH₂Cl₂.
CH3
Br
In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion
to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the
bromonium ion so that a product with anti stereochemistry is formed.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
Br
CH3
H3C
CH3
Create a hydrohalogenation reaction using a 5 carbon alkene illustrating
Markovnikov's rule. Draw and name all reagents
Chapter 7 Solutions
Organic Chemistry
Ch. 7.2 - Write the IUPAC name of each compound.Ch. 7.2 - Write the common name of each alkyne.Ch. 7.5 - Prob. 7.3PCh. 7.7 - Draw a structural formula for a hydrocarbon with...Ch. 7.7 - Hydration of 2-pentyne gives a mixture of two...Ch. 7.9 - Prob. 7.6PCh. 7 - Prob. 7.7PCh. 7 - Show how to prepare each alkyne from the given...Ch. 7 - Prob. 7.9PCh. 7 - Complete each acid-base reaction and predict...
Ch. 7 - Draw structural formulas for the major product(s)...Ch. 7 - Draw the structural formula of the enol formed in...Ch. 7 - Prob. 7.13PCh. 7 - Prob. 7.14PCh. 7 - Prob. 7.15PCh. 7 - Show reagents and experimental conditions you...Ch. 7 - Show reagents and experimental conditions you...Ch. 7 - Show how to convert 1-butyne to each of these...Ch. 7 - Prob. 7.19PCh. 7 - Show reagents and experimental conditions to bring...Ch. 7 - Show reagents to bring about each conversion.Ch. 7 - Propose a synthesis for (Z)-9-tricosene...Ch. 7 - Propose a synthesis of each compound starting from...Ch. 7 - Show how to prepare each compound from 1-heptene....Ch. 7 - Prob. 7.25PCh. 7 - Prob. 7.26PCh. 7 - Following is the structural formula of the...Ch. 7 - The standard procedure for synthesizing a compound...Ch. 7 - Prob. 7.29PCh. 7 - Prob. 7.30PCh. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Prob. 7.35P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.arrow_forwardIdentify the type of reactions involved in each alkene. Predict the major product/s formed in each equation. Draw and name these products.arrow_forwardWhat about the second step in the electrophilic addition of HCl to an alkene-the reaction of chloride ion with the carbocation intermediate? Is this step exergonic or endergonic? Does the transition state for this second step resemble the reactant (carbocation) or the product (alkyl chloride)? Make a rough diagram of what the transition state may look like?arrow_forward
- 10) Synthesis: Make the following products from a suitable cyclic alkene starting material. Look at the functional group PATTERN present in the molecule, including stereochemistry. ♡ Br Brarrow_forwardCH3 CH3 Br- Br2 .CH3 CH2Cl2 CH3 H3C H3C Br Electrophilic addition of bromine, Brɔ, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH,Cl,. In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CH3 CH3 CH3 CH3 H3C H3C :Br: :Br:arrow_forwardWhat is the slow, rate-determining step, in the acid-catalyzed dehydration of 2- butanol? Loss of a b-hydrogen from the carbocation to form an alkene. Protonation of the alcohol to form an oxonium ion. Loss of water from the oxonium ion to form a carbocation. The simultaneous loss of a B-hydrogen and water from the oxonium ion.arrow_forward
- Draw structures for the alkene (or alkenes) that gives the following reaction product. H3C ? H₂/Pd H3C CH₂CH3 CH3arrow_forwardMany insects utilize cyclic ketal structures as pheromones such as the structure shown below. The biosynthetic pathway involves the cyclization of this acetal from the straight chain structure. Draw the straight chain structure that could be used to form this acetal. Use wedges and dashes to correctly depict the stereochemistry. H3C- >arrow_forward10. Draw an energy diagram for the addition of HBr to 1-pentene. Let one curve on your diagram show the formation of 1-bromopentane product and another curve on the same diagram show the formation of 2- bromopentane product. Draw and label the positions for all reactants, intermediates, and products. Which curve has the higher-energy carbocation intermediate? Which curve has the higher-energy first transition state?arrow_forward
- 4) Draw all of the possible alkene products from the following reaction. Indicate in what proportions these alkenes would be produced relative to one another. HO H₂SO4 Refluxarrow_forwardReactions: 20) cis-4-bromo, 3-methyl, 2-pentene undergoes chlorination. Name final product. 21) 3-fluoro-1-cyclohexene undergoes hydrogenation. Name final product. 22) 2,3-dimehtyl-3-hexene undergoes hydration. Draw both products. How many hydrogens in the product? 23) trans-1,2-difluoro-ethene undergoes fluorination. Name final product. 24) 1,4-nonadiene undergoes excessive hydrogenation. How many hydrogens in final product? 25) 1-pentyne undergoes one round of chlorination. Draw product. Now this product undergoes a second round of chlorination. Name final product.arrow_forwardBased on the characteristics of the carbonyl group (C = O), what reactions or transformations take place with aldehydes and ketones? a. nucleophilic additions by oxygenb. electrophilic additions by carbon attackc. nucleophilic additions by carbon attackd. electrophilic substitutions through a carbocationand. acid-base because carbonyl can act as both an electrophile and a nucleophilearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY