
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 7.19P
(a)
Interpretation Introduction
Interpretation:
Reaction mechanism has to be proposed for the given conversion.
(b)
Interpretation Introduction
Interpretation:
The regioselectivity of carbon-carbon bond formation in the given conversion has to be accounted.
Concept Introduction:
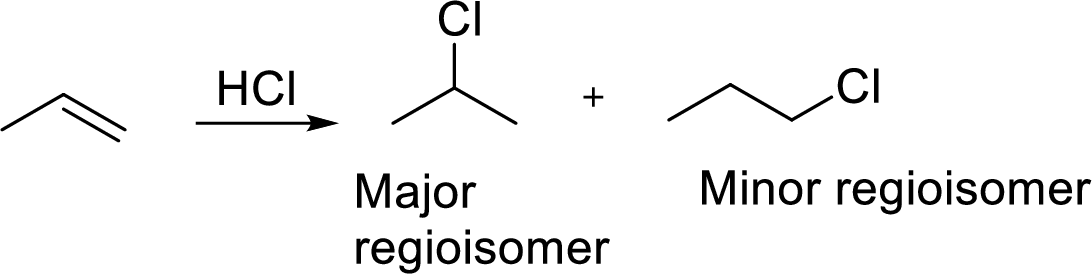
Regioselectivity: It is the favouring of reactants or reagents to bond to one atom or another. Regioisomers are isomers in which connectivity of atoms varies but same number of atoms are present in it. An example of reaction between propene and hydrochloric acid for regioisomers is given as,
(c)
Interpretation Introduction
Interpretation:
For the given conversion, experimental condition has to be described.
(d)
Interpretation Introduction
Interpretation:
For the given conversion, experimental condition has to be described.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The mechanism for acidic hydrolysis of a nitrile resembles the basic hydrolysis, exceptthat the nitrile is first protonated, activating it toward attack by a weak nucleophile (water).Under acidic conditions, the proton transfer (tautomerism) involves protonation on nitrogen followed by deprotonation on oxygen. Propose a mechanism for the acid-catalyzedhydrolysis of benzonitrile to benzamide.
A variation of the acetamidomalonate synthesis can be used to synthesize threonine. The process involves the following steps:
Ethoxide ion deprotonates diethyl acetamidomalonate, forming enolate anion 1;
Enolate anion 1 makes a nucleophilic attack on acetaldehyde, forming tetrahedral intermediate 2;
Protonation of the oxyanion forms alcohol 3;
Acid hydrolysis yields dicarboxyamino alcohol 4;
Decarboxylation leads to the final amino acid.
Write out the mechanism on a separate sheet of paper, and then draw the structure of enolate anion 1.
During a recent synthesis of hispidospermidin, a fungal isolate and an inhibitor of phospholipase C, the investigators employed
employed a novel Friedel-Crafts acylation on a nonaromatic system (J. Am. Chem. Soc. 1998, 120, 4039-4040). The following
acid chloride was treated with a Lewis acid, affording a mixture of two products, 1 and 2. Propose a plausible mechanism for
the formation of compounds 1 and 2.
CI
AICI
进一步农
COCI
2
Part 1
Incorrect. Which of the four mechanistic steps is happening?
Add curved arrow(s) to draw the first step of the mechanism. Modify the given drawing of the products as needed to show
the intermediate that is formed in this step. Use the +/- tools to add/remove charges.
CH₂
H
St
Edit Drawing
H₂C
H
CH₂
CI
Q
M
ΟΣ
Chapter 7 Solutions
Organic Chemistry
Ch. 7.2 - Write the IUPAC name of each compound.Ch. 7.2 - Write the common name of each alkyne.Ch. 7.5 - Prob. 7.3PCh. 7.7 - Draw a structural formula for a hydrocarbon with...Ch. 7.7 - Hydration of 2-pentyne gives a mixture of two...Ch. 7.9 - Prob. 7.6PCh. 7 - Prob. 7.7PCh. 7 - Show how to prepare each alkyne from the given...Ch. 7 - Prob. 7.9PCh. 7 - Complete each acid-base reaction and predict...
Ch. 7 - Draw structural formulas for the major product(s)...Ch. 7 - Draw the structural formula of the enol formed in...Ch. 7 - Prob. 7.13PCh. 7 - Prob. 7.14PCh. 7 - Prob. 7.15PCh. 7 - Show reagents and experimental conditions you...Ch. 7 - Show reagents and experimental conditions you...Ch. 7 - Show how to convert 1-butyne to each of these...Ch. 7 - Prob. 7.19PCh. 7 - Show reagents and experimental conditions to bring...Ch. 7 - Show reagents to bring about each conversion.Ch. 7 - Propose a synthesis for (Z)-9-tricosene...Ch. 7 - Propose a synthesis of each compound starting from...Ch. 7 - Show how to prepare each compound from 1-heptene....Ch. 7 - Prob. 7.25PCh. 7 - Prob. 7.26PCh. 7 - Following is the structural formula of the...Ch. 7 - The standard procedure for synthesizing a compound...Ch. 7 - Prob. 7.29PCh. 7 - Prob. 7.30PCh. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Prob. 7.35P
Knowledge Booster
Similar questions
- Following is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardPhenylacetone can form two different enols.(a) Show the structures of these enols.(b) Predict which enol will be present in the larger concentration at equilibrium.(c) Propose mechanisms for the formation of the two enols in acid and in basearrow_forwardShow how to synthesize the following amines from the indicated starting materials.(a) N-cyclopentylaniline from anilinearrow_forward
- (D)The best route to synthesise aromatic primary amines is by reduction of the corresponding nitro compounds. Draw the reaction scheme for the preparation of p-toluidine.arrow_forwardShow how to synthesize the following amines from the indicated starting materials.(a) N-cyclopentylaniline from aniline (b) N-ethylpyrrolidine from pyrrolidinearrow_forwardA key step in the hydrolysis of acetamide in aqueous acid proceeds by nucleophilic addition of * OH (a) H3O* to CH3Ĉ NH2 (b) H2O to CH3ČNH2 + OH +OH (c) H3O* to CH,ČNH2 (d) HO¯ to CH3CNH2arrow_forward
- Show how to synthesize the following amines from the indicated starting materials byacylation–reduction.(a) N-butylpiperidine from piperidinearrow_forwardPropose a reasonable mechanism to explain the transformation of benzene into acetaminophen. EXPLAIN THE MECHANISM Provide textual explanation about reaction Provide clear handwriting and clear imagearrow_forwardBased on the image attached, it shows methyl salicylate reacts with Ethanamine, and Ether act as a solvent to form N-ethylbenzamide as a product. Explains the mechanism reaction of conversion ester to an amide.arrow_forward
- Several additional amine syntheses are effectively limited to making primary amines. The reduction of azides and nitrocompounds and the Gabriel synthesis leave the carbon chain unchanged. Formation and reduction of a nitrile adds onecarbon atom. Show how these amine syntheses can be used for the following conversions.(a) allyl bromide S allylamine (b) ethylbenzene S p@ethylanilinearrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
