
Concept explainers
The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism.
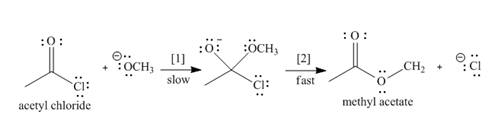
a. Add curved arrows to show the movement of the electrons in each step.
b. Write the rate equation for this reaction, assuming the first step is rate-determining.
c. If the concentration of
d. If the concentrations of both
e. Classify the conversion of acetyl chloride to methyl acetate as an addition, elimination, or substitution.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry (6th Edition)
Additional Science Textbook Solutions
Inorganic Chemistry
Chemistry: Structure and Properties
Organic Chemistry (9th Edition)
Chemistry & Chemical Reactivity
Chemistry: A Molecular Approach
- true or false 1. The rate of a reaction is independent of temperature.2. Slow reactions can be speeded up by raising the temperature.3. Reaction rates increase with increasing temperature.4. Solid catalysts do not affect reaction rates.5. Reactions involving very unstable combinations of chemicals have large rate constants.arrow_forward9. Consider the pair of reactions below to answer the following question(s). КОН a. CH,CH,NH2 CH=CH, heat or КОН CH;CH,OTos CH=CH, heat 1. Which reaction above is faster and why? II. Doubling the concentration of potassium hydroxide in these reactions: a. causes the reaction mechanism to change b. halves the rate of reaction C. has no effect on the rate of reaction d. doubles the rate of reaction IL The mechanism for these reactionsis: a. SN2 b. E2 C. SN1 d. E1 b.arrow_forwardPleasearrow_forward
- What happens to the rate of the following S 2 reaction when the 1-chloropropane is halved and the NaBr is halved? Cl NaBr The rate (Choose one) by a factor of The rate does not change. X S X Śarrow_forwardFind the product of this reaction 1. CH₂O, to c) 2. CH,COOOH ILM 2. H₂O 1. C₂H,MgBr 2. H₂O 3. H₂SO, to HBr, peroxide (CH3) COK, to 2. O 4. H₂O2 Mg. etherarrow_forwardConsider the given reaction in which NC−NC− is the nucleophile and CH3CNCH3CN is the solvent. The reactant molecule has a structure with solid and dashed wedge bonds. A solid wedge () is used to show the bond that is above the plane of the paper, and a dashed wedge () is used to show the bond that is behind the plane of the paper. Draw the product of the following reaction:arrow_forward
- Explain why methyl alcohol reacts with HBr faster than other primary alcohols?arrow_forwardQuestion 15 Consider the mechanism for the conversion below and identify the intermediates that lead to the product. H3O* + CH,OH OH OCH, E O. OH OH HO Only A, B, and D are formed. Only B, C, and E are formed. Only A, C, D, and E are formed. Only A, B, C, and D are formed.arrow_forward6. Write the structure of the product from the first step (Product-1) and then the product from the second step (Product-2) for the two-step reaction sequence shown below. OsO NaHSO 3. Pyridine Intermediate Product-2arrow_forward
- 17. Step 1. Draw the first step of this reaction, starting with the alcohol and SOCl2 Step 2: Draw the second step of this reaction, starting with pyridine and the cation generated in the first step. Step 3: Draw the third step of this reaction, starting with the alkyl chlorosulfite and Cl− .arrow_forward52. In a concerted or single step reaction, A. the rate of forward reaction is equal to the rate of backward reaction. B. the free energy of the reaction is negative C. the free energy of the reaction is positive D. the stability of the transition state controls the rate of the reaction.arrow_forwardBr₂ CH₂Cl2 H₂ Lindlar Catalyst 1. BD3-Et₂0 2. H₂O₂, NaOHarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
