
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 3VCQ
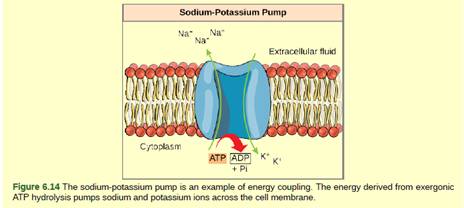
Figure 6.14 The hydrolysis of one ATP molecule releases 7.3 kcal/mol of energy (?G = -7.3 kcal/mol of energy). If it takes 2.1 kcal/mol of energy to move
one Na+ across the membrane (?G = +2.1 kcal/mol of energy), how many sodium ions could be moved by the hydrolysis of one ATP molecule?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You know that the free energy of ATP hydrolysis depends on the ATP/ADP ratio.
Given that under standard conditions DGo = -30.5 kJ/mol, what should be DG of ATP hydrolysis under normal intracellular concentrations?
[ATP] = 2.3 mM, [ADP] = 0.25 mM, [Pi] = 1.65 mM
What is the energy of ATP hydrolysis in a cell that is ATP-depleted?
[ATP] = 0.1 mM, [ADP] = 2.8 mM, [Pi] = 1.65 mM
The average human generates approximately their own weight in ATP every day. A
resting person uses about 25 % of this in ion transport - mostly via the Na*/K*
ATPase. About how many grams of Na* and K* will a sedentary 90-kg person pump
across membranes in a day assuming 25 % of ATP is used to pump Na* and K* via
Na*/K* ATPase?
Each proton that moves across the membrane releases about 14 kJ/mol of energy. Given that ATP requires 30.5 kJ/mol of energy to form, how many protons cross the membrane per ATP synthesized? (Hint: can you have half a proton?)
Chapter 6 Solutions
Biology 2e
Ch. 6 - Figure 6.8 Look at each of the processes shown,...Ch. 6 - Figure 6.10 If no activation energy were required...Ch. 6 - Figure 6.14 The hydrolysis of one ATP molecule...Ch. 6 - Energy is stored long-term in the bonds of and...Ch. 6 - DNA replication involves unwinding two strands of...Ch. 6 - Consider a pendulum swinging. Which type(s) of...Ch. 6 - Which of the following comparisons or contrasts...Ch. 6 - Which of the following is the best way to judge...Ch. 6 - Which of the following is not an example of an...Ch. 6 - In each of the three systems, determine the state...
Ch. 6 - The energy released by the hydrolysis of ATP is...Ch. 6 - Which of the following molecules is likely to have...Ch. 6 - Which of the following is not true about enzymes...Ch. 6 - An allosteric inhibitor does which of the...Ch. 6 - Which of the following analogies best describes...Ch. 6 - Does physical exercise involve anabolic and/or...Ch. 6 - Name two different cellular functions that require...Ch. 6 - Explain in your own words the difference between a...Ch. 6 - Describe the position of the transition state on a...Ch. 6 - Imagine an elaborate ant farm with tunnels and...Ch. 6 - Energy transfers take place constantly in everyday...Ch. 6 - Do you think that the Ea for ATP hydrolysis is...Ch. 6 - With regard to enzymes, why are vitamins necessary...Ch. 6 - Explain in your own words how enzyme feedback...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Professional Application A 30,000-kg freight car is coasting at 0.850 m/s with negligible friction under a hopp...
College Physics
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
1. What are the main organs of the skeletal system?
Human Anatomy & Physiology
What is anatomical position?
Human Anatomy & Physiology (2nd Edition)
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (6th Edition) - standalone book
QUANTITATIVE Punnett Squares as Genetic Tools. The genetic characters of seed color (where Y is the allele for ...
Becker's World of the Cell (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- (3.7) Suppose the intracellular pH of an alkaliphile is 8.5 but the extracellular environment is at pH9.5. The cellular membrane of the bacterium has an electric potential of Af = -190 mV. How many c-subunits must the alkaliphilic ATP synthase have for it to work as an ATP synthase rather than proton pump? Assume that the free energy change for the phosphorylation of ADP to ATP is AFp = +50 kJ/mol. Answer:arrow_forwardyeasts are able to produce high internal concentrations of glycerol to counteract the osmotic pressure of the surrounding media. suppose that a sample of yeast cells were placed in a 4% sodium chloride solution by weight. The density of solution is at 25 C = 1.02 g/ml, Molecular weight of solute = 58.44 g/mol, i of glycerol = 1 and R=0.08205 L-atm/mol-K What is the weight of solute in grams What is the moles of solute What is the volume of the solution in liters What is the molarity of the solution What is the value of the temperature to be used to solved for the osmotic pressure of the solution What is the osmotic pressure of solutionarrow_forwardAt different glucose concentrations (2 mol of glucose, 4 mol of glucose, 8 mol of glucose), at what time, in milliseconds, was the increase in ATP the greatest and how does this affect the potential (mV) graph? I am having hard time analyzing this thank youarrow_forward
- In the reaction ATP + glucose → ADP + glucose-6-phosphate, ΔG° is -16.7 kJ/mol. Assume that both ATP and ADP have a concentration of 1 M and T = 25°C. What ratio of glucose-6- phosphate to glucose would allow the reverse reaction to occur?arrow_forwardIn the situations described below, what is the free energy change if 1 mole of Na* is transported across a membrane from a region where the concentra- tion is 1 µM to a region where it is 100 mM? (Assume T = 37 °C.) (a) In the absence of a membrane potential. (b) When the transport is opposed by a membrane potential of 70 mV. (c) In cach case, will hydrolysis of 1 mole of ATP suffice to drive the trans- port of 1 mole of ion, assuming pH 7.4 and the following cytoplamic concentrations: ATP= 4.60 mM, P = 5.10 mM, ADP = 310 µM?arrow_forwardMagnesium ion (Mg2+) forms complexes with the negative charges of the phosphate in ATP. In the absence of Mg2+, would ATP have more, less, or the same stability as when the ion is present?arrow_forward
- Twenty-three milligrams of glucose were eaten by the bacteria Sanacoccus pumasareus. Calculate the hypothetical amount of ATP your patient can generate under aerobic respiration with this amount of glucose. (Note: Glucose MW-180.16 g/mole; 1 mole= 6.02 x 1023 molecules (Avogadro's number)). 2.8 x 10^24 ATPs 02.9 x 10^21 ATPs 028 x 10-21 ATP5 029 x 10 24 ATPS Lacks information, cannot be determinedarrow_forwardHow many net ATPs would be produced if the following fatty acid is completely oxidized intocarbon dioxides and water? 94.5 96.5 98 98.5 108arrow_forwardGastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calculate the amount of free energy required to concentrate the H+ in 1 L of gastric juice at 37 °C. Under cellular conditions,how many moles of ATP must be hydrolyzed to provide this amount of free energy? The free-energy change for ATP hydrolysis under cellular conditions is about −58 kJ/mol . Ignore the effects of the transmembrane electrical potential.arrow_forward
- if an 8.4-oz (250 mL) energy drink can has 27 grams of sugar, calculate the moles of ATP produced in the body when 1 oz of the drink is consumed assuming all the sugars in 1 oz of the drink are composed of only glucose.arrow_forwardAssume that mitochondria contain 0.23 Molar KCL and 0.010 Molar NaCl. Calculate the grams per liter, of a carbohydrate similar to glucose with a molecular weight 240 amu, that will have the same osmolarity as the mitochondria.. Calculate answer to four decimal places, do not specify units in the answer.arrow_forwardIn an experiment, a 0.001 (mole fraction) solution of polysaccharide in water is made and is placed in the compartment A (see Figure below). Compartment B is filled with pure water. The two compartments are separated by a porous semi-permeable membrane that allows the exchange of water molecules between the two compartments, but not that of the larger polysaccharide molecules a) Show that the chemical potential of water in compartment A is lower than that in compartment B by 2.48 J/mol. b) As a result of this chemical potential difference, water molecules will move from compartment B to compartment A. This causes the pressure in compartment A, relative to that in B, to increase. How would this affect the chemical potential of water in compartment B? When would the diffusion of water from B to A cease (i.e. equilibrium is achieved)? c) Using your answer to part (b), work out the difference between the pressure in compartment A and B when…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax
Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY